Published online Dec 14, 2008. doi: 10.3748/wjg.14.7075
Revised: November 14, 2008
Accepted: November 21, 2008
Published online: December 14, 2008
AIM: To investigate if an immune imbalance may account for the development and progression of chronic radiation enteritis. We analyzed the Th1/Th2 immune response profile early and 6 mo after fractionated colorectal irradiation.
METHODS: A rat model of fractionated colorectal γ-irradiation (4-Gy fractions, 3 fractions per week) was designed to investigate the effects of cumulative dose on inflammatory mediators (cytokines and chemokines) and immune response (Th1/Th2 profile and immunosuppressive mediator IL-10) during acute (early) response and 6 mo after the end of fractionated irradiation (chronic response). Analyses were performed 1 d after the cumulative doses of 16 Gy and 36 Gy and 1 d, 3 d, and 26 wk after the cumulative dose of 52 Gy.
RESULTS: Without causing histological damage, fractionated radiation induced elevated expression of IL-1β, TNFα, MCP-1, and iNOS in distal colonic mucosa during the early post-irradiation phase. At that time, a Th2 profile was confirmed by expression of both the Th2-specific transcription factor GATA-3 and the chemokine receptor CCR4 and by suppression of the Th1 cytokine IFNγ/IP-10 throughout the irradiation protocol. After 6 mo, despite the 2-fold reduction of iNOS and MCP-1 levels, the Th2 profile persisted, as shown by a 50% reduction in the expression of the Th1 transcription factor T-bet, the chemokine receptor CCXCR3, and the IFNγ/STAT1 pathway. At the same time-point, the immunosuppressive IL-10/STAT3 pathway, known to regulate the Th1/Th2 balance, was expressed, in irradiated rats, at approximately half its level as compared to controls. This suppression was associated with an overexpression of SOCS3, which inhibits the feedback of the Th1 polarization and regulates IL-10 production.
CONCLUSION: Colorectal irradiation induces Th2 polarization, defective IL-10/STAT3 pathway activation and SOCS3 overexpression. These changes, in turn, maintain a immunological imbalance that persists in the long term.
- Citation: Grémy O, Benderitter M, Linard C. Acute and persisting Th2-like immune response after fractionated colorectal γ-irradiation. World J Gastroenterol 2008; 14(46): 7075-7085
- URL: https://www.wjgnet.com/1007-9327/full/v14/i46/7075.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7075
| Gene | Forward primer | Reverse primer |
| IL-1β | CAACAAAAATGCCTCGTGC | TGCTGATGTACCAGTTGGG |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| MCP-1 | CAGCCAGATGCAGTTAATGCC | AGCCGACTCATTGGGATCAT |
| INOS | GATTTTTCACGACACCCT | GGTCCTCTGGTCAAACTC |
| IL-10 | GTTGCCAAGCCT-TGTCAGAAA | TTTCTGGGCCATGGTTCTCT |
| IFN-γ | CACGCCGCGTCTTGGT | TCTAGGCTTTCAATGAGTGTGCC |
| Suppressors of cytokine signalling proteins | ||
| SOCS3 | CCTCCAGCATCTTTGTC-GGAAGAC | TACTGGTCCAGGAACTCCCGAATG |
| Transcription factors | ||
| T-Bet | TCCTGTCTCCAGCCGTTTCT | CGCTCACTGCTCGGAACTCT |
| GATA-3 | GGCGGCGAGATGGTACTG | TCTGCCCATTCATTTTATGGTAGA |
| Chemokine receptors | ||
| CXCR3 | AGGTCAGTGAACGTCAAGTGCTAG | CAAAAAGAGGAGGCTGTAGAGGA |
| CCR4 | GCCTCCAAGACAGACTTCCTTG | AGCGTTCGGTTCTAGTTTCCAC |
| Housekeeping | ||
| HPRT | GCTCGAGATGTCATGAAGGAGA | TCAGCGCTTTAATGTAATCCAGC |
During radiation therapy for pelvic or abdominal cancer, the intestines are a critical dose-limiting organ. Despite precautions in treatment planning (e.g. multiple fields) and delivery, patients develop radiation-induced bowel injury during and after therapy[1]. Radiation enteropathy therefore remains an important obstacle to the radiocurability of abdominal tumors and continues to impair patients’“quality of life”. Symptoms of acute bowel toxicity occur among about 80% of these patients, and include vomiting, diarrhea, hemorrhages and ulcerations due to the direct effects of radiation on the intestinal mucosa[2]. Explanations based on recognition that irradiation induces changes in cellular functions have recently replaced the concept attributing the severity of acute intestinal radiation toxicity to disruption of the epithelial barrier and mucosal inflammation.
Acute (early) effects are those observed during the course of radiation therapy: they are usually transient and cease shortly after its completion. In some patients, persistent acute damage causes consequential effects to appear later on, i.e. after radiation exposure[3]. These delayed effects, called chronic radiation enteritis, concern fewer patients (50%) but are important clinically because of their chronic progressive nature and their significant long-term morbidity. Delayed radiation enteropathy typically presents, from the clinical standpoint, 6 mo to 3 years after irradiation. It is characterized by dysmotility and malabsorption, sometimes developing into fibrosis and eventually, in some cases, bowel obstruction, years or even decades after radiation exposure. A latency period was previously thought to exist between the time of radiation treatment and the onset of radiation-induced damage, but many studies now show that this is not the case. Experimental evidence indicates that the onset of delayed radiation effects is a continuous process that starts immediately at irradiation[4]. The process includes the production of cytokines and reactive oxygen species (ROS), which cause responses in the surrounding tissue, including cell infiltration. These “waves” of response may be interpreted as the result of failed attempts at adaptation and then later as the evidence of dysregulated tissue response. Recent studies show that irradiation induces the synthesis of various cytokines in several tissues, including the intestines[5] and lungs[6]. These cytokines lead to cell infiltration and fibroblast stimulation, thus enhancing collagen synthesis[7]. In addition, cytokine production by immune cells is crucial to immune response to infectious agents and disease prevention.
Many diseases, including inflammatory bowel disease[8], are associated with imbalances between Th1 (T helper cell type 1) and Th2 (T helper cell type 2) cytokines. Because these cell subpopulations tend to function antagonistically towards each another, the persistence of disease susceptibility and resistance depends on the profiles of the cytokines that each type secretes. Several reports show that ionizing radiation induces the preferential differentiation of Th cells into Th2 cells in the spleen[9,10] and more recently, in the intestines[11], where this Th2 dominance is characterized by repression of IFNγ. Th2 cells play a critical role in the pathogenesis of radiation-induced pneumonitis, which precedes lung fibrosis[12].
Th1 and Th2 cells were originally distinguished from each other by their specific cytokine expression profiles. In vivo analysis of these polarized Th cells has revealed differential sets of molecular expression, including specific chemokine/chemokine receptors. Typically, the chemokine receptor CXCR3 is expressed exclusively on Th1 lymphocytes, which migrate to the inflammatory sites in response to CXCR3 ligands and interferon-inducible protein (IP)-10[13,14]. Likewise, CCR4 is preferentially expressed on Th2 cells[15]. Specific expression of transcription factors leads to differential expression of polarized Th cells. For example, T-bet, expressed specifically in Th1 cells, mediates IFNγ production, while GATA-binding protein 3 (GATA-3), which suppresses this production, is thought to be the most important factor associated with the development of the Th2 phenotype and inhibition of the Th1 phenotype[16]. Th1 cytokines can activate macrophages, regulate cell-mediated immune responses, and promote tumoricidal activity. Th2 cells, in contrast, promote humoral immunity. Each Th subset mutually inhibits the growth and function of the other one. Members of the suppressor of cytokine signaling (SOCS) family of proteins are described as feedback inhibitors of a broad range of cytokine signaling pathways, regulating the amplitude and duration of the polarization influenced by T-bet or GATA-3[17]. Notably, SOCS3 inhibits the signal transduction pathway implicating IFNγ[18].
Determining Th1/Th2 balance during the radiotherapy protocol and, in particular, its long-term balance requires a longitudinal study. In this study, a rat model of fractionated colorectal-irradiation was designed to investigate the effects of cumulative dose on the inflammatory mediators and the immune response. Our study suggests that the downregulation of Th1-type cells, induced by γ-irradiation, persists for at least 6 mo after the end of the irradiation protocol. This supports the hypothesis that the radiation-induced impairment of inflammation control mechanisms plays a critical role in both acute and late effects.
Adult male Wistar rats (Elevage Janvier, France) weighing 200-250 g were housed (three per cage) with food and water ad libitum. All experiments were conducted in accordance with the French Ministry of Agriculture regulations for animal experimentation (No. 2001-464, May 2001).
Anesthetized rats were sham-irradiated or exposed to a γ-ray source (60Co, 1 Gy/min). The radiation field was confined to the colorectum (field size: 2 by 2.5 cm). Radiation was delivered 3 times a week (4-Gy fractions) for a total dose of 16, 36, or 52 Gy. The cumulative doses of 16 and 36 Gy correspond nearly to one third and two thirds of the total fractionated dose (52 Gy), respectively. Radiation response was assessed on the first day after the cumulative dose of 16 and 36 Gy, and day 1 and day 3 of the cumulative dose of 52 Gy, and 26 wk after it reached 52 Gy.
After the rats were anesthetized, distal colon tissue in the irradiation field was excised and rinsed with saline. Whole-tissue samples were collected and fixed with 4% formaldehyde for immunostaining assays, while scraped mucosa layers were snap-frozen and then stored at -80°C for RNA extraction.
The mRNA levels of inflammation-related cytokines and chemokines and of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) were measured by real-time polymerase chain reaction (RT-PCR). Total RNA was extracted from the colon mucosa samples with the RNeasy kit (Qiagen). RNA purity and integrity were checked by spectrophotometric analysis and agarose gel electrophoresis. In accordance with the manufacturer’s instructions, 1 μg of total RNA was reverse-transcribed into cDNA with random hexamers and the SuperScript II RNase H (GibcoBRL) in a 20-μL reaction volume. SYBR chemistry (Applied Biosystems) was used to amplify PCR in the ABI-Prism 7000 detection system (Applied Biosystems), under the following conditions: 50°C for 2 min, 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 1 min. The primer sequences, designed with Primer Express software (Applied Biosystems), are listed in Table 1.
Total proteins were obtained by colon homogenization in a cold RIPA buffer (Sigma) containing a standard protease-inhibitor cocktail. Protein concentrations of cytoplasmic and nuclear extracts were measured with a modified Bradford method from Biorad Laboratories (Biorad, France). The samples were then stored at -80°C.
Proteins (25 μg) were boiled in SDS and mercapto-ethanol buffer and then separated on a 120 g/L polyacrylamide gel (NuPAGE gels; Invitrogen, France) and electroblotted. Polyvinylidene difluoride membranes were incubated with a blocking solution (50 g/L skimmed milk in TPBS containing 1 mL/L Tween 20), washed with TPBS and incubated with rabbit polyclonal STAT1 p84/p91 (1/700, Santa Cruz), STAT3 (1/300, Santa Cruz) and SOCS3 (1/200, Santa Cruz) for 1 h at room temperature. After washing, immunodetection was performed with the respective horseradish-linked secondary antibodies (1/1000; Santa Cruz). GAPDH protein was detected similarly with a rabbit anti-GAPDH polyclonal antibody (1/1000, Santa Cruz) to verify uniformity in gel loading. Chemiluminescence was detected according to the manufacturer’s protocol (ECL, Biorad). Mean band densities were quantified with a Las 3000 apparatus (Fugifilm) and normalized to the total amount of protein in the control.
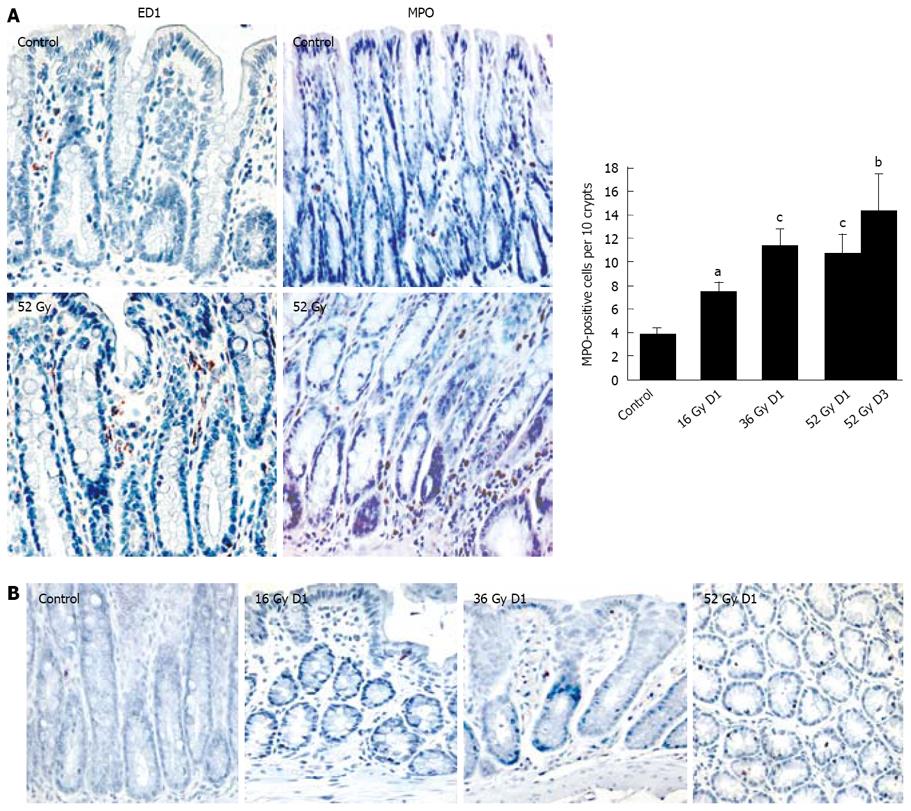
Neutrophils and macrophages: Fixed distal colon specimens were dehydrated, embedded in paraffin and then sectioned into 5 μm thick slices. After dewaxing in xylene and rehydration by exposure to graded ethanols, sections were processed to reveal immunoreactivity to myeloperoxidase (MPO), a marker of neutrophils, or were prepared for macrophage detection. All sections were subjected to an endogenous peroxidase blocking solution (3% H2O2). For macrophage staining only, the slides were boiled in 10% citrate buffer for antigen retrieval. To reduce non-specific binding, all slides were pre-incubated with the protein blocker (Dako) before treatment for 1 hour at 26°C with anti-rat MPO antibody (NCL-MYELOp; 1:300, Novocastra) or at 37°C with an antibody binding the activated macrophage marker ED1 (MCA341; 1:50, Serotec). Secondary reagents were a secondary antibody, followed by the Vector Elite ABC kit (Dako) for neutrophil detection, and the LSAB 2 system HRP kit (Dako) for macrophages. Whenever necessary, slides were washed with a Tris buffer (50 mmol/L Tris-HCl; 0.3 mol/L NaCl; 0.1% Tween 20; pH 7.6). For both the primary and secondary staining, sections were treated with a NovaRED kit (Vector Laboratories Inc.) for color development, and then counterstained with Mayer’s hemalum. For MPO staining, positive cells were counted under a light microscope in a blinded fashion and expressed as the mean number of marked cells per ten random crypts (100 crypts).
Apoptotic cell detection: Apoptotic cells were visualized by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick-end labeling assay (TUNEL) using the In Situ Cell Death Detection kit (Roche Molecular Biochemicals, France) according to the manufacturer’s instructions. Briefly, deparaffinized and rehydrated tissue sections were incubated in proteinase K (20 mg/L in 10 mmol/L Tris-HCl, pH 7.6) for 10 min at 37°C. Sections were exposed to the TUNEL reaction mixture at 37°C for 1 h. After washing in PBS buffer, POD (peroxidase) was added to react for 30 min at 37°C. For both stainings, the slides were treated with a NovaRED™ kit (Vector Laboratories Inc, Burlingame, CA) for colour development and counterstained with Meyer’s hemalun.
For the real-time PCR, we used the comparative ΔΔCT-method for the relative mRNA quantification of target genes, normalized to the reference gene HPRT, and the relevant sham-irradiated sample. Specifically, we determined 2-ΔΔCT, with ΔΔCT the difference between ΔCT(irradiated sample) and ΔCT(sham-irradiated sample) and ΔCT the difference between the mean CT(gene of interest) and the mean CT(HPRT reference gene). CT is the threshold cycle of fluorescence intensity. Each experimental group consisted of 6 animals. Values of PCR and MPO-positive cell counts are expressed as the mean ± SE.
All data are expressed as the mean ± SE for 6 animals. Data were analyzed by one-way ANOVA followed by a Bonferroni test to determine the significance of the differences.
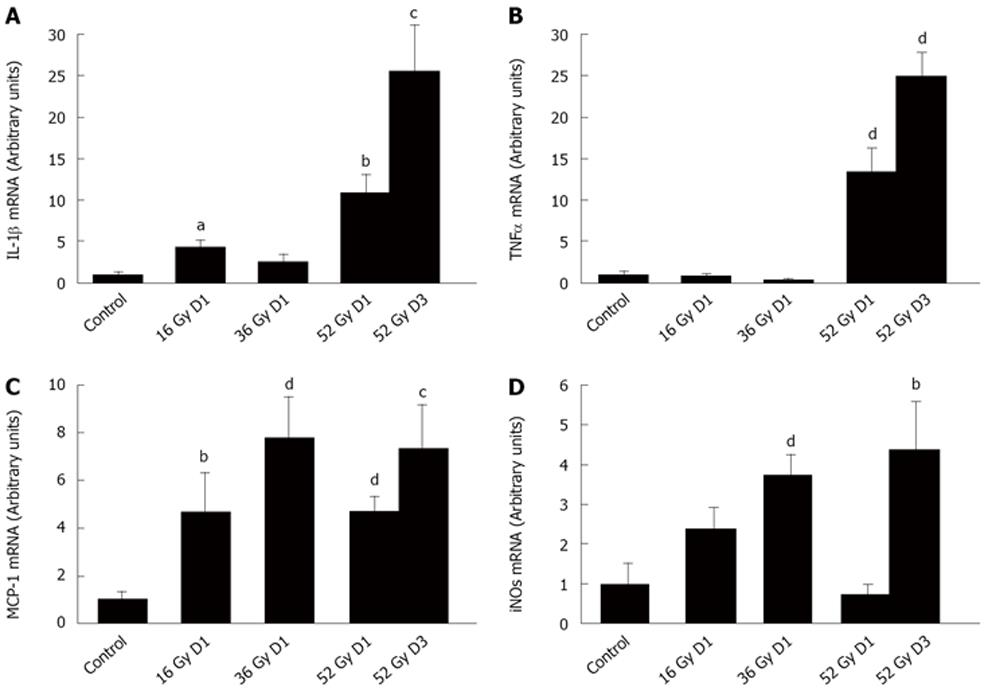
Overexpression of pro-inflammatory cytokines is often a sign of the onset of inflammation and has been characterized as an early effect of intestinal radiation[5]. To test whether fractionated irradiation induces a persistent and progressive inflammatory process, we assessed the expression of inflammatory mediators, relative to the housekeeping gene HPRT, in rat colon mucosa on D1 in irradiated rats in all 3 dose groups (16, 36 and 52 Gy) and on D3 in the 52 Gy group. IL-1β expression increased by a factor of 4.5 (P < 0.05) on D1 after colorectal γ-irradiation to 16-Gy exposure, compared with controls. On the other hand, 24 h after the 36-Gy cumulative dose, IL-1β expression did not differ from that in the sham-exposed rats. Finally, after the 52-Gy dose, IL-1β mRNA rose to levels dramatically higher than in controls (11-fold, P < 0.01) on D1 and still higher (25-fold, P < 0.005) on D3 (Figure 1A). TNFα expression after the total 52-Gy dose was also 13.5 times higher than in controls on D1 (P < 0.005) and remained elevated at D3 (P < 0.001) (Figure 1B). Similarly, the level of MCP-1, known to recruit and activate monocytes and macrophages in tissue, increased significantly during the fractionated protocol (Figure 1C). In addition, the expression of iNOS, strongly secreted by macrophages, remained high throughout the protocol (Figure 1D).
Histological analysis of distal colons showed no distinct difference between the sham-irradiated group and the rats with cumulative doses of 16, 36, and 52 Gy. Immunostaining of macrophages showed an increase in ED1-positive macrophages in the lamina propria on D1 and D3 after the maximal cumulative dose of 52 Gy (Figure 2A). This macrophage accumulation may be related to the overexpression of TNFα, MCP-1 and iNOS. In addition, the number of MPO-positive neutrophils was nearly twice as high as in controls on D1 after the 16-Gy exposure (P < 0.01) and remained elevated throughout the protocol, about 3 times higher than in controls in the 36-Gy group (P < 0.001) and in the 52-Gy groups on D1 and D3 (Figure 2A). In addition, TUNEL assay showed no significant modification of the apoptotic cell number during fractionated irradiation (Figure 2B).
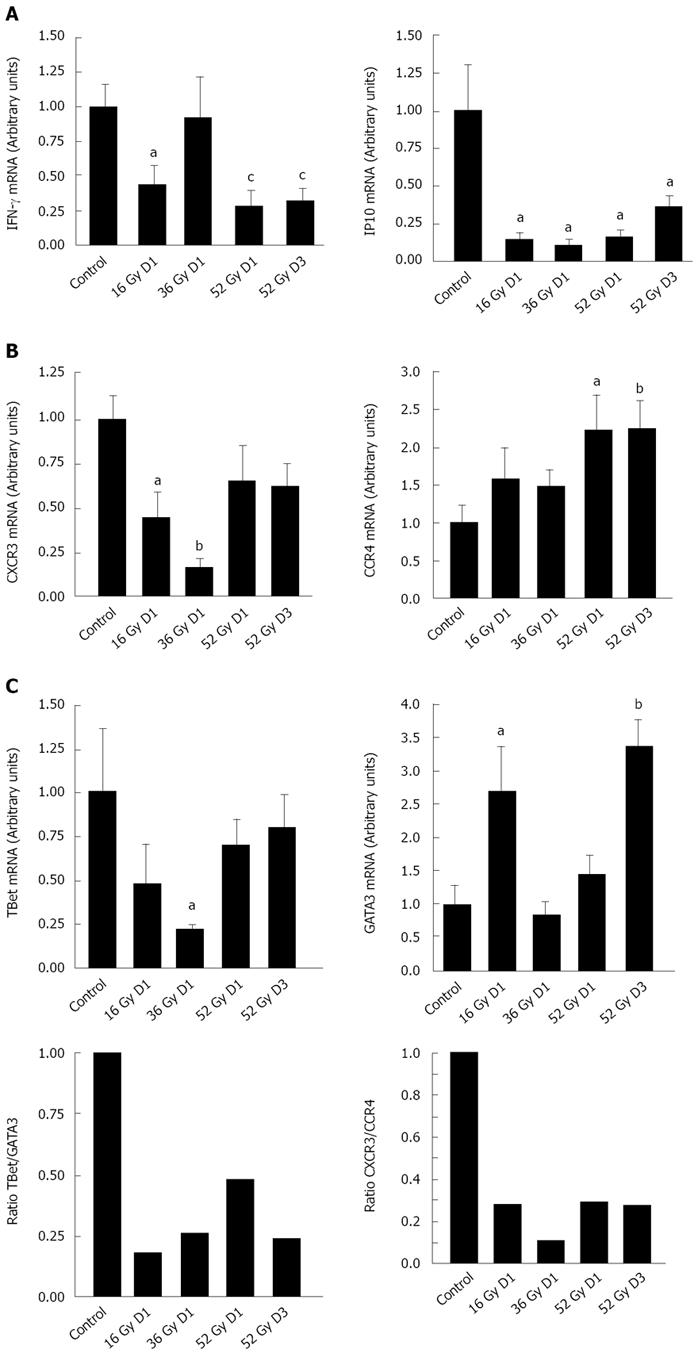
As previously reported[11], abdominal irradiation initiated a Th2-cell immune response characterized by suppression of IFNγ expression. Here too, we observed that fractionated irradiation induced a wave suppressing IFNγ expression, reducing it significantly (3-fold decrease, P < 0.005) (Figure 3A). This decrease was associated with reduction of IFN-inducible genes by the end of the protocol, including IFNγ-inducible 10 kDa protein (IP-10) (P < 0.05).
We measured Th cell populations present in the mucosa during the fractionated protocol more directly by taking into account the fact that activated Th cells acquire and maintain high levels of specific patterns of chemokine receptors: CXCR3 is thus a marker of Th1 and CCR4 of Th2[15]. Fractionated irradiation modified the chemokine receptor profile (Figure 3B). CXCR3 levels fell by 50% (P < 0.05) after a 16-Gy cumulative dose and 80% (P < 0.01) after a 36-Gy dose. The CXCR3 level remained low after a cumulative dose of 52 Gy, but CCR4 expression increased significantly (P < 0.01). The CXCR3/CCR4 ratio, which serves as an indicator of the Th1/Th2 balance[15], fell about 70% during the course of treatment, thus confirming that irradiation induced Th2 dominance. Further confirmation comes from analysis of the transcription factors T-bet and GATA-3, respectively, selectively expressed in Th1 and Th2 cells. The T-bet profile closely resembled that of CXCR3 (Figure 3C). GATA-3 expression increased more than T-bet expression did at every period, and the ratio of T-Bet to GATA-3 ratio fell, decreasing 4-fold by the end of the protocol.
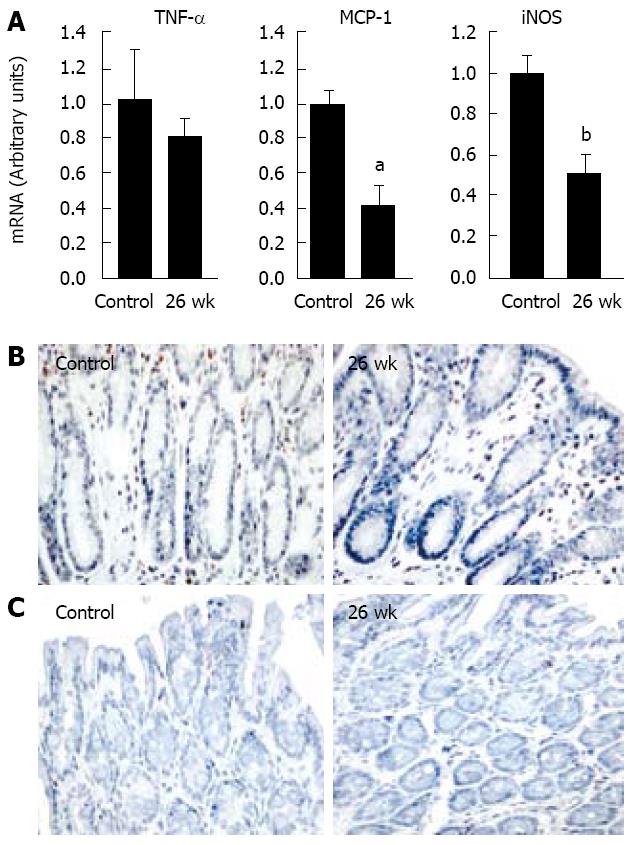
Histological analysis of the colon 26 wk after the last delivered fraction showed no changes. To investigate the inflammatory response, we analyzed the expression of mediators related to macrophage infiltration. No change in TNFα expression was observed during this period. Surprisingly, however, expression of MCP-1 and iNOS decreased significantly, by 60 and 50% respectively. No increase in macrophage infiltration and no significant modification of apoptotic cell umber were observed at 6 mo (Figure 4).
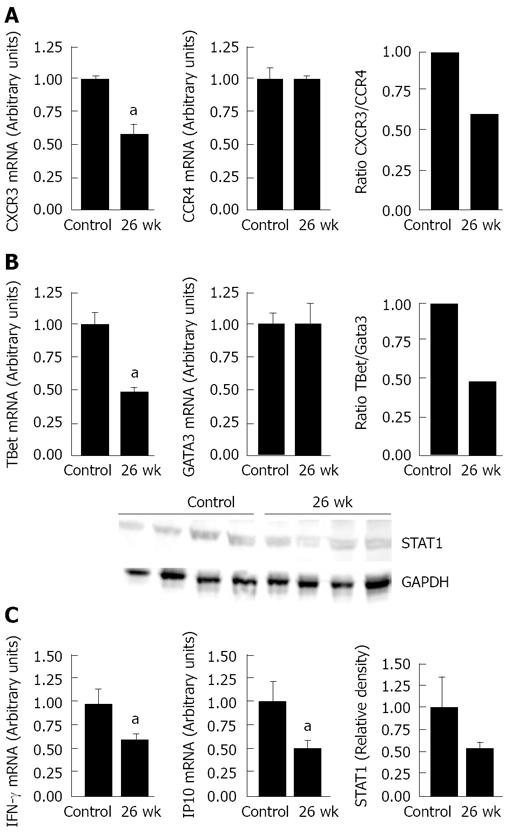
The fractionated schedule may increase the probability that radiation-induced inflammation will become chronic and/or induce damage at intermediate times post-irradiation. No previous report has prospectively identified changes in the Th1/Th2 balance according to expression of chemokine receptors and transcription factors over time after irradiation. Analysis of the Th1 profile showed a significant 2-fold reduction (P < 0.05) in both CXCR3 and T-bet expression compared with the controls 26 wk after the end of the protocol (Figure 5A and B). Levels of CCR4 and GATA3 (both Th2 markers) did not change.
The consequence of the Th1/Th2 imbalance was seen in the significant reduction in IFNγ and IP-10 (P < 0.05): expression of both fell by half. Because IFNγ delivers signals through the STAT family of signal transducers, such as STAT1, we sought to determine if reduced IFNγ expression in irradiated mucosa was correlated with a reduction in STAT1. Western blot analysis showed that STAT1 immunoreactivity was also lower at 26 wk than in controls (Figure 5C). Taken together, these data indicate that the shift towards Th2 dominance is maintained 26 wk after irradiation.
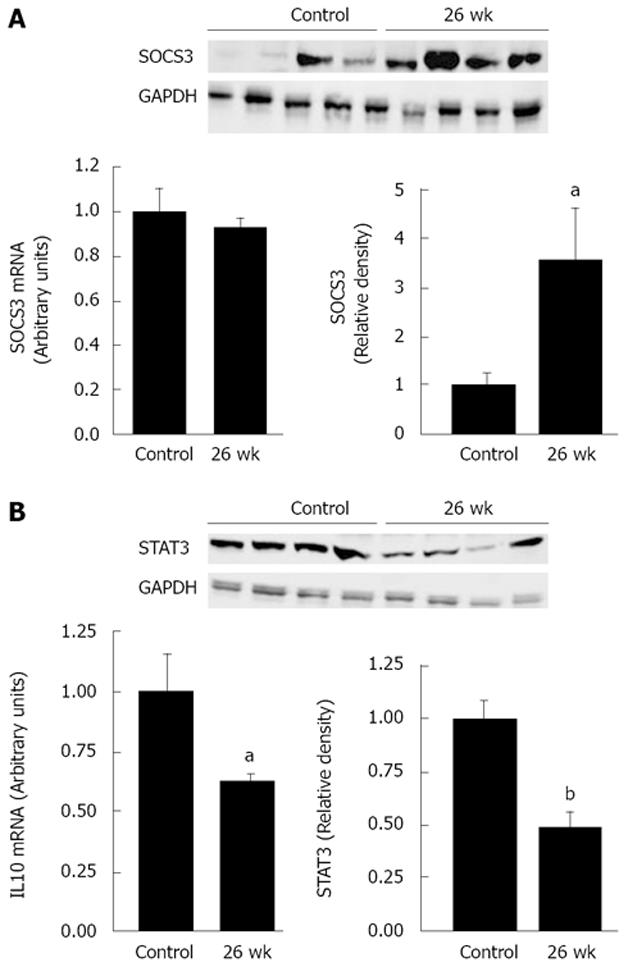
The relative decrease of STAT1 protein levels prompted us to investigate the possible role of the SOSC proteins, which inhibit STAT signaling. A particularly interesting candidate from this family is SOCS3, known to interfere with the IFNγ/Stat-1 pathway[19,20]. Western blot analysis showed the SOCS3 protein levels remained high at 26 wk after irradiation (P < 0.05) (Figure 6A). Because SOCS3 regulates production of the immunoregulatory cytokine IL-10 by modulating STAT3 signaling[21], we assessed IL-10 and STAT3 expression (Figure 6B). IL-10 expression (P < 0.05) 26 wk after the end of the protocol was half as much compared to the control rats and was correlated with a similar decrease in STAT3 protein levels (P < 0.001). These data show that elevated SOCS3 levels modify expression of IL-10 and STAT3.
The pathological process of radiation injury begins immediately after radiation exposure, but its clinical and histological features may not become apparent for weeks, months, or even years after treatment. In this study, we investigated whether fluctuations in cytokine levels after radiation treatment continue over a relatively long period 6 mo after the end of treatment, and whether they predict late effects. Identifying the critical factors could provide useful tools for selecting patients for treatment to prevent late damage or to reduce its severity. If we knew how early cytokine responses modify downstream late effects, we might be able to target cytokines for prevention. The results showed that each dose fraction influenced tissue inflammatory responses and led to long-term persistence of the immune imbalance.
Fractionated radiation produces a series of repeated insults to healthy tissues: these lead to inflammatory events that do not dissipate within 24 h, so that repetitive responses accumulate over the period of radiation therapy[22]. During our fractionated protocol in the rat, we identified inflammatory reactions in the colon induced by a cascade of inflammatory mediators, including IL-1β, TNFα, MCP-1, and iNOS, with levels and latency periods specific for each mediator. In addition, histological observations showed infiltration of inflammatory cells (macrophages and neutrophils). TNFα is known to be a key mediator of pathogenesis in a broad range of infectious and predominantly Th-mediated inflammatory diseases, possibly via macrophage induction of iNOS and thus NO synthesis. However, iNOS has also been shown to enhance induction of TNFα synthesis[23], contributing to local tissue destruction during the chronic inflammation that is one of the direct consequences of inflammatory processes. A previous study found a positive correlation between iNOS activity levels and disease severity in patients with ulcerative colitis, but this association is less clear in Crohn’s disease[24]. The involvement of iNOS-derived NO in acute radiation syndrome is suggested by an increase in iNOS gene expression or enzyme activity in the intestines[25]. The present study revealed a biphasic pattern of iNOS expression: early overexpression during the fractionated protocol, followed by delayed suppression, seen at 6 mo. In particularly, iNOS regulates chemokine expression involved in leukocyte (especially neutrophil) trafficking: the absence of iNOS enhances lung inflammatory responses, associated with increased production of MCP-1 by endothelial cells and macrophages[26]. Reports about iNOS regulation of the production of this CC chemokine are contradictory. In vitro studies show that inhibition of endogenous NO synthesis by a NOS inhibitor increases MCP-1 levels in endothelial cells[27], whereas, in a rat model of pulmonary granulomatous inflammation, inhibition of NO production reduced MCP-1 expression[28]. Our results are consistent with the hypothesis that iNOS can regulate MCP-1 expression, because increased levels of iNOS were associated with both increased neutrophil recruitment and MCP-1 expression during the fractionated treatment. Moreover, at a later phase, the expression of both iNOS and MCP-1 was cut in half. At this time, no explanation is given, but additional attention is being placed on macrophages, the essential iNOS producers that are inactivated with regard to inflammatory mediator production. A more general suppression is achieved with IL-10, but exposure to IL-4 or related cytokines initiates a so called “alternatively activated macrophage” or M2 macrophages[29]. These cells have distinct functional properties that integrate them into polarized type 2 responses, tissue remodeling and repair. These cells show diminished capacity to produced iNOS. Furthermore, the maturation stage of the macrophage population may be worth considering in the later phase.
The immunomodulatory activity of iNOS is reported to influence Th cell development and to downregulate the induction of Th1 responses, thereby promoting a Th2 response[30]. Multiple pathways appear to be involved in initiating the type 2 T-cell responses. Gu et al[31] reported an impaired Th2 response in MCP-1 knockout mice, thus demonstrating that MCP-1 is necessary for the generation of Th2 cells. A possible feedback loop for Th2 activation might be the production of IL-4 and IL-13 by Th2 cells: these cytokines stimulate MCP-1 production and lead to further recruitment of Th2 cells[13]. Our results corroborate previously published work reporting on the initiation of a Th2 response in the early phase of abdominal radiation[11]. In that study, we identified a Th2 response during the fractionated protocol. It included suppression of the Th1 cytokine profile, that is, of both IFNγ and the IFN-inducible gene IP-10. These are also identified by the specific pattern of chemokine receptors (CXCR3 and CCR4) and the specific transcription factors (T-bet and GATA-3), selectively expressed in Th1 cells and Th2 cells, respectively, that control the differentiation and the functions of these Th cell subsets. The correlation between the suppression of CXCR3 and T-bet observed in the present study also corroborates a previous report[32] that deletion of T-bet reduces CXCR3 expression: CXCR3 is both a direct transcription target of T-bet and the chemokine receptor of IP-10. IFNγ may act upstream of T-bet to drive CXCR3 expression[32]. Consequences of T-bet deletion include the failure of primary CD4+ T cells generated under Th1 polarizing conditions to migrate to the site of inflammation because of defects in several specific mechanisms of the T-cell trafficking pathway. Surprisingly, suppression of CXCR3 and T-bet continued during the later phase, for reasons that we could not determine.
An essential role of STAT1 is the activation of Th1 production of T-bet and IFNγ. Analysis of the expression of IFNγ and STAT1 showed that the persistent suppression of the IFNγ/IP-10 was correlated with low STAT1 protein levels at 6 mo. Taken together, the low ratios of CXCR3/CCR4 and Tbet/Gata3, as well as the defective IFNγ/STAT1 expression, indicate a prolonged Th2 dominance 6 mo after irradiation. Experiments with STAT1 knockout mice have demonstrated the crucial importance of this protein for macrophage activation and NK cytolytic activity in vivo: as a consequence, these mice have elevated susceptibility to viral and bacterial infections[33,34]. The differentiation of the IFNγ-producing Th1 cells is crucial for the resistance to intracellular infections, notably by “alternatively activated macrophage” inactivation.
Some reports describe families of cytokines that induce inhibition of the STAT1 signal cascade, such as SOCS. SOCS3 impairs IFNγ-induced STAT1-dependent gene activation[35]. We recently showed that acute irradiation induces SOCS3 overexpression[36]. In this study, immunoblot analysis showed that the protein levels of SOCS3 remained high in the later post-irradiation phase. Berlato et al[37] reported that constitutive expression of SOCS3 diminishes the quantities of TNFα and NO produced by the transduced cells in response to LPS stimulation. Crespo et al[38] confirmed that SOCS3 inhibits transcription of the iNOS gene, apparently by suppressing interaction between STAT1α and IFNγ. SOCS3 might also block the signaling pathways required to activate the posttranscriptional mechanisms that regulate TNFα synthesis[39]. Thus, the low levels of MCP1 and iNOS and the unchanged amount of TNFα observed 6 mo after irradiation suggest a possible role for SOCS3 in the suppression of these genes.
Previous studies report discovered that IL-10 rapidly induces SOCS3 in a STAT3-dependent manner[40,41]. Because SOSC3 can negatively regulate responses to different activating cytokines and bacterial products, it is speculated that the anti-inflammatory action of IL-10 is due to induction of SOCS3[42]. Here, however, our analysis of the expression of IL-10/STAT3 signaling shows that the irradiation-induced SOCS3 overexpression was instead associated with suppression of IL-10 mRNA and STAT3 protein. Berlato et al[37] found previously that constitutive expression of SOCS3 can block the capacity of IL-10 to activate STAT3, and Kinjyo et al[43] showed that STAT3 is hyperactivated in SOCS3-deficient T cells during T cell differentiation.
The development and persistence of chronic inflammatory disorders such as inflammatory bowel disease can be induced by defects in IL-10 production or in STAT3 signaling molecules or by overexpression of SOCS3[44]. Accordingly, suppression of T-cell expression of SOCS3 and the identification of this protein intracellular target may be the mechanisms to introduce tolerance and thus prevent the immune dysregulation induced by radiotherapy protocols. These results raise the question whether the presence of Th2 immune cells in the intestines after irradiation (acute and delayed) may result from changes in the dynamics of lymphocyte recirculation, involving the alteration of the microvasculature and the presence of differential chemokines.
The use of radiation therapy to treat cancer inevitably involves exposure of normal tissues. Gastrointestinal symptoms after pelvic radiotherapy, which affect quality of life, are substantially more common than generally recognised and are frequently poorly managed. Patients may experience symptoms associated with damage to normal tissue during the course of abdominal therapy for a few weeks after therapy or months or years later. In fact, it is not known what venet occurring at an early stage may predispose to significant changes later on.
In this article, it is shown that immune mechanism alterations may assume greater importance during the early irradiation. Experimentally carried out in the rat model, fractionated irradiation modified the T helper cell polarization, as shown by Th1 cytokine (IFNγ) repression in the colon. This shift occurred via transcriptional regulation of the level of cytokine mediators, via modification of specific signal transducers of IFN signalling and a through the secretion of a feed-back inhibitor of Th1 polarization, thus potentiating the Th2 profile.
The major result is that the immunity alteration persisted in long term, i.e. after the end of the radiotherapy protocol. These data raise the question whether the post-irradiation Th2 polarization in the intestine (both acute and delayed) may result from changes in the dynamics of lymphocyte recirculation.
Although the irradiation-induced immune damage has not yet been studied in great detail, a good understanding of this immunity alteration and the relationship between acute and late effects may motivate clinicians to look more often at methods of decreasing tissue toxicity, thus contributing to ameliorate the patents’ quality of life after radiotherapy. In radiotherapy, priority needs to be given to assessing simple methods of preventing bowel toxicity, without compromising the control of the tumor.
The paper is technically well done and well written and presents novel data.
Peer reviewers: Elke Cario, MD, Division of Gastroenterology and Hepatology, University Hospital of Essen, Institutsgruppe I, Virchowstr. 171, Essen D-45147, Germany; Samuel B Ho, MD, Chief, Gastroenterology Section (111D), VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, United States
S- Editor Tian L L- Editor Negro F E- Editor Ma WH
| 1. | Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529-536. [Cited in This Article: ] |
| 2. | Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol). 2007;19:790-799. [Cited in This Article: ] |
| 3. | Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223-231. [Cited in This Article: ] |
| 4. | McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. A sense of danger from radiation. Radiat Res. 2004;162:1-19. [Cited in This Article: ] |
| 5. | Linard C, Marquette C, Mathieu J, Pennequin A, Clarencon D, Mathe D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427-434. [Cited in This Article: ] |
| 6. | Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421-1427. [Cited in This Article: ] |
| 7. | Strup-Perrot C, Mathe D, Linard C, Violot D, Milliat F, Francois A, Bourhis J, Vozenin-Brotons MC. Global gene expression profiles reveal an increase in mRNA levels of collagens, MMPs, and TIMPs in late radiation enteritis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G875-G885. [Cited in This Article: ] |
| 8. | MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. [Cited in This Article: ] |
| 9. | Park HR, Jo SK, Paik SG. Factors effecting the Th2-like immune response after gamma-irradiation: low production of IL-12 heterodimer in antigen-presenting cells and small expression of the IL-12 receptor in T cells. Int J Radiat Biol. 2005;81:221-231. [Cited in This Article: ] |
| 10. | Han SK, Song JY, Yun YS, Yi SY. Effect of gamma radiation on cytokine expression and cytokine-receptor mediated STAT activation. Int J Radiat Biol. 2006;82:686-697. [Cited in This Article: ] |
| 11. | Gremy O, Benderitter M, Linard C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after gamma-irradiation in the rat by modulating the cytokine pattern. World J Gastroenterol. 2006;12:4996-5004. [Cited in This Article: ] |
| 12. | Westermann W, Schobl R, Rieber EP, Frank KH. Th2 cells as effectors in postirradiation pulmonary damage preceding fibrosis in the rat. Int J Radiat Biol. 1999;75:629-638. [Cited in This Article: ] |
| 13. | Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123-128. [Cited in This Article: ] |
| 14. | Garcia-Lopez MA, Sanchez-Madrid F, Rodriguez-Frade JM, Mellado M, Acevedo A, Garcia MI, Albar JP, Martinez C, Marazuela M. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest. 2001;81:409-418. [Cited in This Article: ] |
| 15. | Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129-134. [Cited in This Article: ] |
| 16. | Ritz SA, Cundall MJ, Gajewska BU, Swirski FK, Wiley RE, Alvarez D, Coyle AJ, Stampfli MR, Jordana M. The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo. Clin Exp Immunol. 2004;138:213-220. [Cited in This Article: ] |
| 17. | Langberg CW, Hauer-Jensen M, Sung CC, Kane CJ. Expression of fibrogenic cytokines in rat small intestine after fractionated irradiation. Radiother Oncol. 1994;32:29-36. [Cited in This Article: ] |
| 18. | Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181-3187. [Cited in This Article: ] |
| 19. | Federici M, Giustizieri ML, Scarponi C, Girolomoni G, Albanesi C. Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. J Immunol. 2002;169:434-442. [Cited in This Article: ] |
| 20. | Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kuhbacher T, Hamling J, Folsch UR, Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379-385. [Cited in This Article: ] |
| 21. | Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021-1031. [Cited in This Article: ] |
| 22. | Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex ‘wound’. Radiother Oncol. 2002;63:129-145. [Cited in This Article: ] |
| 23. | Huang FP, Niedbala W, Wei XQ, Xu D, Feng GJ, Robinson JH, Lam C, Liew FY. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur J Immunol. 1998;28:4062-4070. [Cited in This Article: ] |
| 24. | Guihot G, Guimbaud R, Bertrand V, Narcy-Lambare B, Couturier D, Duee PH, Chaussade S, Blachier F. Inducible nitric oxide synthase activity in colon biopsies from inflammatory areas: correlation with inflammation intensity in patients with ulcerative colitis but not with Crohn's disease. Amino Acids. 2000;18:229-237. [Cited in This Article: ] |
| 25. | Freeman SL, Hossain M, MacNaughton WK. Radiation-induced acute intestinal inflammation differs following total-body versus abdominopelvic irradiation in the ferret. Int J Radiat Biol. 2001;77:389-395. [Cited in This Article: ] |
| 26. | Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murphy ME, Murphy HS, Ward PA. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol. 2003;163:2319-2328. [Cited in This Article: ] |
| 27. | Desai A, Miller MJ, Huang X, Warren JS. Nitric oxide modulates MCP-1 expression in endothelial cells: implications for the pathogenesis of pulmonary granulomatous vasculitis. Inflammation. 2003;27:213-223. [Cited in This Article: ] |
| 28. | Setoguchi K, Takeya M, Akaike T, Suga M, Hattori R, Maeda H, Ando M, Takahashi K. Expression of inducible nitric oxide synthase and its involvement in pulmonary granulomatous inflammation in rats. Am J Pathol. 1996;149:2005-2022. [Cited in This Article: ] |
| 29. | Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23-35. [Cited in This Article: ] |
| 30. | Lawrence CE, Paterson JC, Wei XQ, Liew FY, Garside P, Kennedy MW. Nitric oxide mediates intestinal pathology but not immune expulsion during Trichinella spiralis infection in mice. J Immunol. 2000;164:4229-4234. [Cited in This Article: ] |
| 31. | Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407-411. [Cited in This Article: ] |
| 32. | Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432-3439. [Cited in This Article: ] |
| 33. | Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431-442. [Cited in This Article: ] |
| 34. | Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol. 2000;165:3571-3577. [Cited in This Article: ] |
| 35. | Ekchariyawat P, Pudla S, Limposuwan K, Arjcharoen S, Sirisinha S, Utaisincharoen P. Burkholderia pseudomallei-induced expression of suppressor of cytokine signaling 3 and cytokine-inducible src homology 2-containing protein in mouse macrophages: a possible mechanism for suppression of the response to gamma interferon stimulation. Infect Immun. 2005;73:7332-7339. [Cited in This Article: ] |
| 36. | Linard C, Gremy O, Benderitter M. Reduction of peroxisome proliferation-activated receptor gamma expression by gamma-irradiation as a mechanism contributing to inflammatory response in rat colon: modulation by the 5-aminosalicylic acid agonist. J Pharmacol Exp Ther. 2008;324:911-920. [Cited in This Article: ] |
| 37. | Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404-6411. [Cited in This Article: ] |
| 38. | Crespo A, Filla MB, Murphy WJ. Low responsiveness to IFN-gamma, after pretreatment of mouse macrophages with lipopolysaccharides, develops via diverse regulatory pathways. Eur J Immunol. 2002;32:710-719. [Cited in This Article: ] |
| 39. | Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M, Davis R, Flavell R, Kollias G. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760-3770. [Cited in This Article: ] |
| 40. | Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J Immunol. 2003;170:1383-1391. [Cited in This Article: ] |
| 41. | Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567-576. [Cited in This Article: ] |
| 42. | Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563-573. [Cited in This Article: ] |
| 43. | Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021-1031. [Cited in This Article: ] |