Published online Nov 7, 2008. doi: 10.3748/wjg.14.6299
Revised: October 30, 2008
Accepted: November 6, 2008
Published online: November 7, 2008
Ghrelin causes interdigestive contractions of the stomach in rats. However, it remains unknown whether ghrelin causes interdigestive contractions in the small intestine. Four strain gauge transducers were implanted on the antrum, duodenum, proximal and distal jejunum. After an overnight fast, gastrointestinal (GI) contractions were recorded in freely moving conscious rats. Spontaneous phase III-like contractions were observed at every 13-16 min in rat GI tract. The fasted motor patterns were replaced by the fed motor pattern immediately after food intake. Two minutes after finishing the spontaneous phase III-like contractions in the antrum, acyl ghrelin (0.8, 2.4 and 8.0 μg/kg per min) was continuously infused for 30 min. Three-five minutes after the starting ghrelin infusion, augmented phase III-like contractions were observed at the antrum, duodenum, and jejunum. Ghrelin infusion (0.8, 2.4 and 8.0 μg/kg per min) significantly increased motility index of phase III-like contractions at the antrum and jejunum in a dose dependent manner, compared to that of saline injection. Thus, it is likely that exogenously administered ghrelin causes phase III-like contraction at the antrum, which migrates to the duodenum and jejunum. The possible role of 5-HT, in addition to ghrelin, in mediating intestinal migrating motor complex (MMC), is discussed.
- Citation: Taniguchi H, Ariga H, Zheng J, Ludwig K, Takahashi T. Effects of ghrelin on interdigestive contractions of the rat gastrointestinal tract. World J Gastroenterol 2008; 14(41): 6299-6302
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6299.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6299
In the interdigestive state, the stomach and small intestine show a remarkable motor pattern, known as the migrating motor complex (MMC)[1]. MMC consists of three phases; phaseI(period of motor quiescence), phase II (period of irregular low amplitude contractions) and phase III (period of regular high amplitude contractions). In humans and dogs, MMC is usually observed every 90-120 min in the interdigestive state. In contrast, in rats, MMC cycle is less than 20 min and not so regular, compared to humans and dogs[2,3]. Exogenously administered motilin does not induce phase III-like contractions in rats. Motilin or its receptors are not found in rats[4].
Ghrelin, a 28-amino acid peptide, was discovered as the endogenous ligand for growth hormone secretagogue receptor (GHS-R) from the rat stomach[5]. Because of a structural resemblance to motilin, ghrelin is known as the motilin-related peptide[6,7]. Ghrelin administration causes phase III-like contraction at the antrum and duodenum in conscious rats[3,8]. We recently showed that gastric spontaneous phase III-like contractions were abolished by ghrelin receptor antagonists[9]. This suggests that endogenous ghrelin regulates spontaneous phase III-like contractions of the rat stomach.
However, it still remains unknown whether ghrelin regulates intestinal phase III-like contractions in rats. In the current study, we investigated whether exogenously administered ghrelin stimulates phase III-like contractions of gastrointestinal (GI) tract in conscious rats.
Male Sprague-Dawley rats weighing 280-340 g were kept in-group cages under conditions of controlled temperature (22-24°C), humidity and light (12 h light cycle starting at 7:00 am) with free access to laboratory chow and water. Protocols describing the use of rats were approved by the Institutional Animal Care and Use Committee of Zablocki VA Medical Center (Milwaukee) and carried out in accordance with the National Institute of Health “Guide for the Care and Use of Laboratory Animals”. All efforts were made to minimize animal suffering and to reduce the number of animal in experiments.
After overnight fasting, the rats were anesthetized with intraperitoneal injection of pentobarbital sodium (45 mg/kg). Through a midline laparotomy, strain gauge transducers were implanted on the serosal surface of the antrum, duodenum and jejunum. Duodenal transducers were implanted at 5 cm distal from the pylorus. Jejunal transducers were implanted at 15 cm (the proximal jejunum: J-1) and 25 cm (the distal jejunum: J-2) distal from the pylorus, respectively. The wires from transducer were exteriorized through abdominal wall, ran under skin toward the back. Intravenous catheter was inserted into right jugular vein, and similarly exteriorized to the back, as previously reported[2,9]. The catheter was filled with heparinized saline (100 U/mL) to prevent coagulation. Wires and a catheter were protected by a protective jacket (Star Medical, Tokyo, Japan). After the surgery, rats were housed individual and were allowed to recover for one week before the experiments.
After the implantation of transducers, rats were given food once daily at 12:00 pm-16:00 pm, as previously reported[2]. Experiments of GI motility recording were started at 9:00 am every day. The wires from the transducer were connected to the recording system (Power-Lab model 8SP, ADI instruments, Colorado Springs, CO). GI contractions were measured with free access to water in freely moving conscious rats. Spontaneous phase III-like contractions were observed for 2-3 h. Phase III-like contractions were defined as clustered potent contractions with amplitude of more than 4 g, as previously reported[10].
Fujino et al[3] reported that bolus injection of acyl ghrelin (1 g/rat; iv) induced phase III-like contraction in conscious rats. In general, bolus injection of certain peptides abruptly increased its plasma level. In our previous study, acyl ghrelin (0.8 μg/kg per min) was continuously infused for 5 min and potent phase III-like contractions were observed in the antrum in rats[9]. In our current study, acyl ghrelin (0.8, 2.4 and 8.0 μg/kg per min) was continuously infused for 30 min. Acyl ghrelin was purchased from Tocris Cookson (Ellisville, MO).
Motility index (MI), area under the curve, was calculated using a computer-assisted system (PowerLab, ADInstruments, Colorado Springs, CO). MI in GI tract was compared thirty minutes before and during the infusion of acyl ghrelin. Saline infused rats served as controls.
Results were shown as mean ± SE. ANOVA followed by student’s t-test was used to assess the difference among groups. A P value < 0.05 was considered to be statistically significant.
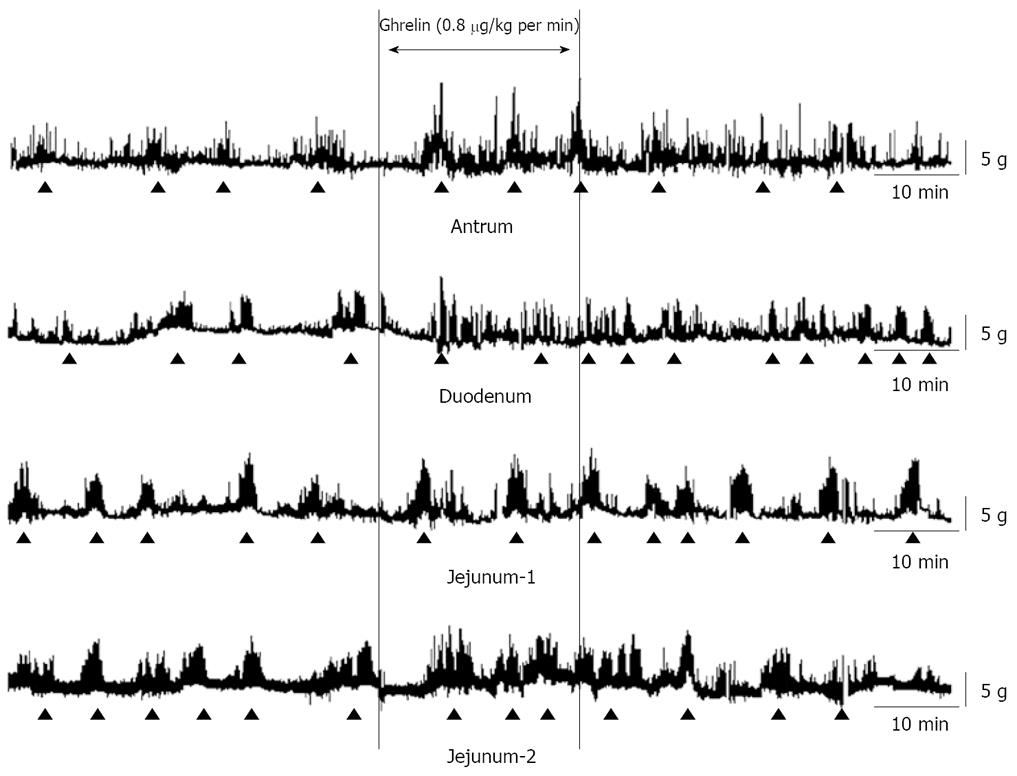
It has been showed that spontaneous phase III-like contractions are observed at 12-15 min intervals of the stomach[3,9,10] in conscious rats. In our current study, cyclic changes of contractions were detected in the antrum, duodenum, J-1 and J-2 including a quiescence period (phaseI-like contractions) followed by a grouping of strong contractions (phase III-like contractions). Spontaneous phase III-like contractions were observed at every 13-16 min in rat GI tract. The fasted motor patterns were replaced by the fed motor pattern immediately after food intake[11].
Two minutes after finishing the spontaneous phase III-like contractions in the antrum, acyl ghrelin (0.8, 2.4 and 8.0 μg/kg per min) was continuously infused for 30 min. Three-five minutes after the starting ghrelin infusion, augmented phase III-like contractions were observed at the antrum, duodenum, J-1 and J-2 (Figure 1).
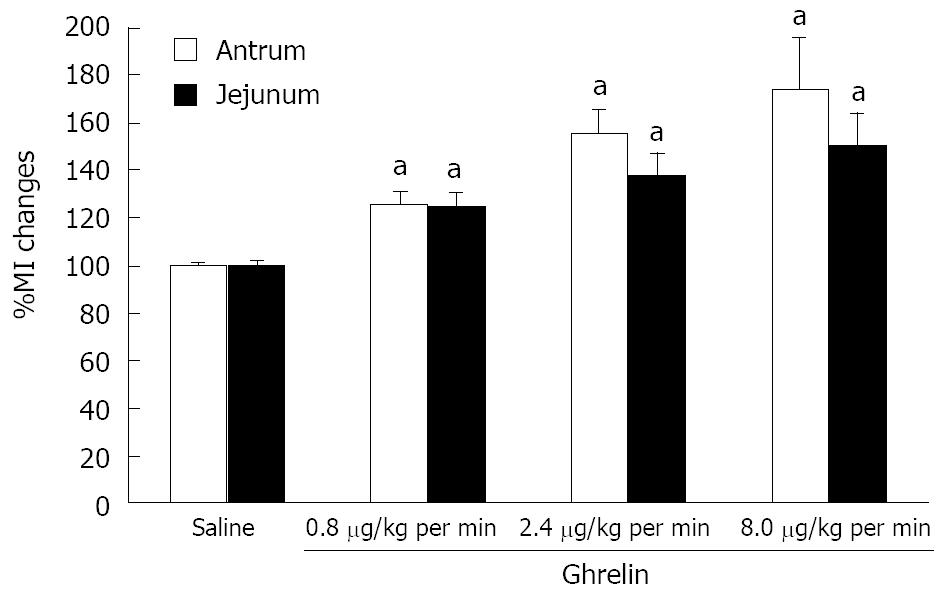
Ghrelin infusion (0.8, 2.4 and 8.0 μg/kg per min) significantly increased MI of phase III-like contractions at the antrum and jejunum compared to that of saline injection, in a dose-dependent manner (Figure 2).
It is well established that exogenously administered ghrelin causes phase III-like contractions in the interdigestive state at the antrum and duodenum in rats[3]. However, it is not clear whether intestinal phase III-like contractions are affected by ghrelin administration.
We evaluated the effects of peripherally infused ghrelin on gastrointestinal phase III-like contractions in freely moving conscious rats. We demonstrated that ghrelin infusion induced phase III-like contractions in the antrum, duodenum, proximal and distal jejunum in a dose-dependent manner (0.8-80 μg/kg per min). This suggests that gastric phase III-like contractions induced by exogenously administered ghrelin migrate distally to the small intestine.
We have previously shown that GHS-R antagonists significantly inhibited spontaneous phase III-like contractions in conscious rats[9], suggesting that endogenously released ghrelin regulates spontaneous phase III-like contractions. We also showed the correlation between the plasma ghrelin levels and occurrence of gastric phaseI- and III-like contractions of the antrum[9]. However, it is not clear whether intestinal phase III-like contractions are regulated by endogenously released ghrelin. Previous report showed that ghrelin stimulates motility in the rat small intestine and that the stimulatory effect of ghrelin is mediated via cholinergic neurons of the myenteric plexus[12].
Our recent study showed that GHS-R antagonists inhibited phase III-like contractions at the antrum, but not the duodenum and the jejunum[11]. Previous studies also showed that GHS-R antagonists did not affect phase III-like contractions in the duodenum[8].
It is likely that exogenously administered ghrelin (a pharmacological dose of ghrelin) causes phase-III like contraction at the antrum, which migrates to the jejunum. In contrast, endogenously released ghrelin causes spontaneous phase III-like contractions at the antrum, which do not migrate to the small intestine.
It has been demonstrated that 5-HT is involved in mediating interdigestive contractions of the small intestine in rats[13]. Subcutaneous or intravenous administration of 5-HT can induce intestinal migrating myoelectrical activity in rats[14,15]. Intestinal migrating myoelectrical activity was reduced by a 5-HT3 antagonist, but not by a 5-HT4 antagonist in conscious rats[15]. However, others showed that intestinal migrating myoelectrical activity was reduced by a 5-HT4 antagonist, as well as a 5-HT3 antagonist[16].
Our recent study showed that phase III-like contractions at the jejunum, not the antrum and duodenum, were significantly attenuated by 5-HT4 antagonists. In contrast, 5-HT3 antagonists did not affect phase III-like contractions in all of upper GI tract[11]. These suggest that spontaneous phase III-like contractions at the jejunum is mediated via 5-HT4 receptors, but not 5-HT3 receptors.
5-HT3 receptors[17] and 5-HT4 receptors[18] are located on the cholinergic neurons of the myenteric plexus as well as sensory neurons of the intestinal mucosa[19]. Nerve endings of sensory neurons may well be the targets for the 5-HT released from enterochromaffin (EC) cells[20]. It is generally accepted that 5-HT stimulates intrinsic nerve fibers via 5-HT4 receptors[21], while 5-HT stimulates extrinsic nerve fibers via 5-HT3 receptors[19,22] in rats.
5-HT3 receptors are located on the nerve terminal of vagal afferent of the duodenal mucosa in rats[23]. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4 receptors on sensory CGRP neurons of the rat colon in vitro[24]. These suggest that 5-HT4 receptors play a major role in mediating an intrinsic neural reflex. It is conceivable that luminally released 5-HT from duodenal EC cells initially stimulates duodenal phase III-like contractions via 5-HT4 receptors located on intrinsic primary afferent neurons (IPAN).
It has been shown that ghrelin receptors are synthesized in vagal afferent neurons and transported to the afferent terminal. This is the major pathway conveying ghrelin signals for starvation and growth hormone secretion to the brain[25]. Blockade of the gastric vagal neuron by vagotomy or perivagal application of capsaicin abolished ghrelin-induced feeding, GH secretion, and activation of NPY-producing and GHRH-producing neurons[25]. Ghrelin-induced acid secretion is also abolished by bilateral vagotomy[26].
Our recent study showed that spontaneous phase III-like contractions were completely disappeared in vagotomized rats[11]. These results suggest that ghrelin-induced spontaneous phase-III like is mediated via vagal pathways.
Spontaneous phase III-like contractions are mainly regulated by ghrelin in the antrum, while spontaneous phase III-like contractions are regulated by 5-HT in the jejunum. Released ghrelin from the gastric mucosa initiates gastric phase III-like contractions via vagal dependent pathways. Released 5-HT from intestinal EC cells induces intestinal phase III-like contractions via IPAN in rats.
S- Editor Xiao LL E- Editor Ma WH
| 1. | Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol. 1969;217:1757-1763. [Cited in This Article: ] |
| 2. | Ariga H, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Fixed feeding potentiates interdigestive gastric motor activity in rats: importance of eating habits for maintaining interdigestive MMC. Am J Physiol Gastrointest Liver Physiol. 2008;294:G655-G659. [Cited in This Article: ] |
| 3. | Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227-240. [Cited in This Article: ] |
| 4. | Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160-168. [Cited in This Article: ] |
| 5. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [Cited in This Article: ] |
| 6. | Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, Alexander G, Chenard MP, Rio MC. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology. 2000;119:395-405. [Cited in This Article: ] |
| 7. | Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337-345. [Cited in This Article: ] |
| 8. | Wang Y, Dong L, Cheng Y, Zhao P. Effects of ghrelin on feeding regulation and interdigestive migrating complex in rats. Scand J Gastroenterol. 2007;42:447-453. [Cited in This Article: ] |
| 9. | Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675-680. [Cited in This Article: ] |
| 10. | Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R862-R872. [Cited in This Article: ] |
| 11. | Taniguchi H, Ariga H, Zheng J, Ludwig K, Mantyh C, Pappas TN, Takahashi T. Endogenous ghrelin and 5-HT regulate interdigestive gastrointestinal contractions in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G403-G411. [Cited in This Article: ] |
| 12. | Edholm T, Levin F, Hellstrom PM, Schmidt PT. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25-30. [Cited in This Article: ] |
| 13. | Pineiro-Carrero VM, Clench MH, Davis RH, Andres JM, Franzini DA, Mathias JR. Intestinal motility changes in rats after enteric serotonergic neuron destruction. Am J Physiol. 1991;260:G232-G239. [Cited in This Article: ] |
| 14. | Sagrada A, Brancaccio N, Schiavone A. 5-Hydroxytryptamine affects rat migrating myoelectric complexes through different receptor subtypes: evidence from 5-hydroxytryptophan administration. Life Sci. 1990;46:1207-1216. [Cited in This Article: ] |
| 15. | Lordal M, Hellstrom PM. Serotonin stimulates migrating myoelectric complex via 5-HT3-receptors dependent on cholinergic pathways in rat small intestine. Neurogastroenterol Motil. 1999;11:1-10. [Cited in This Article: ] |
| 16. | Axelsson LG, Wallin B, Gillberg PG, Sjoberg B, Soderberg C, Hellstrom PM. Regulatory role of 5-HT and muscarinic receptor antagonists on the migrating myoelectric complex in rats. Eur J Pharmacol. 2003;467:211-218. [Cited in This Article: ] |
| 17. | Miyata K, Kamato T, Nishida A, Ito H, Yuki H, Yamano M, Tsutsumi R, Katsuyama Y, Honda K. Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther. 1992;261:297-303. [Cited in This Article: ] |
| 18. | Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358:2061-2068. [Cited in This Article: ] |
| 19. | Gershon MD. Review article: roles played by 5-hydro-xytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13 Suppl 2:15-30. [Cited in This Article: ] |
| 20. | Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl). 1995;191:203-212. [Cited in This Article: ] |
| 21. | Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281-1290. [Cited in This Article: ] |
| 22. | Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine (5-HT) on the discharge of vagal mechanoreceptors and motility in the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:51-59. [Cited in This Article: ] |
| 23. | Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217-226. [Cited in This Article: ] |
| 24. | Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol. 1996;270:G778-G782. [Cited in This Article: ] |
| 25. | Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120-1128. [Cited in This Article: ] |
| 26. | Yakabi K, Ro S, Onouhi T, Tanaka T, Ohno S, Miura S, Johno Y, Takayama K. Histamine mediates the stimulatory action of ghrelin on acid secretion in rat stomach. Dig Dis Sci. 2006;51:1313-1321. [Cited in This Article: ] |