Published online Oct 14, 2008. doi: 10.3748/wjg.14.5893
Revised: August 26, 2008
Accepted: September 2, 2008
Published online: October 14, 2008
AIM: To explore the diffusion gradient b-factor that optimizes both apparent diffusion coefficient (ADC) measurement and contrast-to-noise (CNR) for assessing tumor response to transarterial chemoembolization (TACE) in a rabbit model.
METHODS: Twelve New Zealand white rabbits bearing VX2 tumors in the liver were treated with TACE. Diffusion-weighted imaging (DWI) with various b values was performed using the same protocol before and 3 d after treatment with TACE. ADC values and CNR of each tumor pre- and post-treatment with different b factors were analyzed. Correlation between ADC values and extent of necrosis in histological specimens was analyzed by a Pearson’s correlation test.
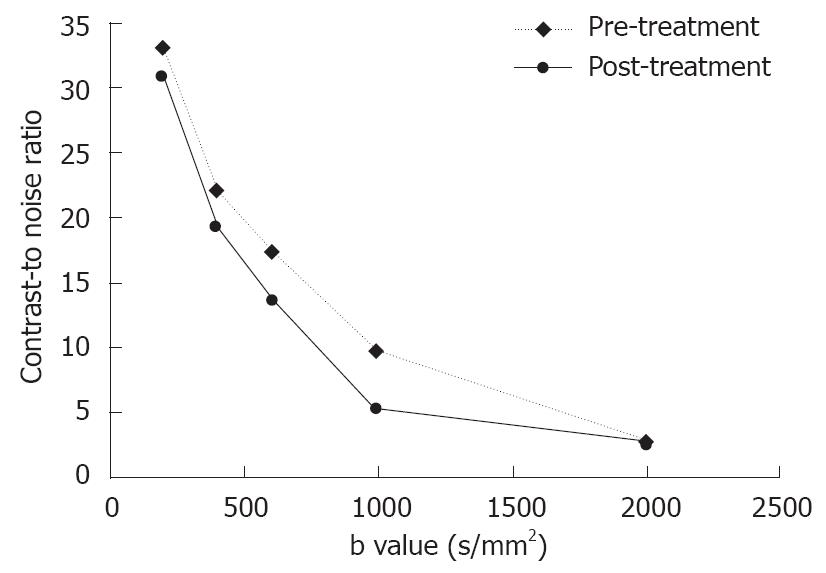
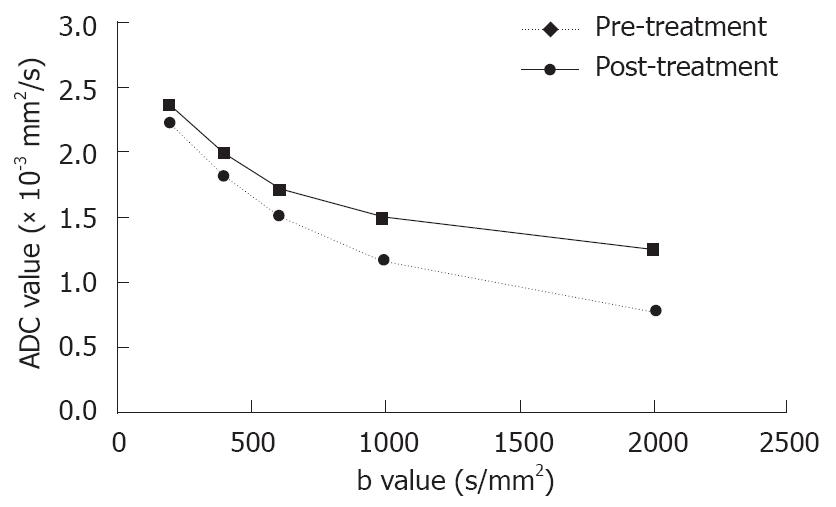
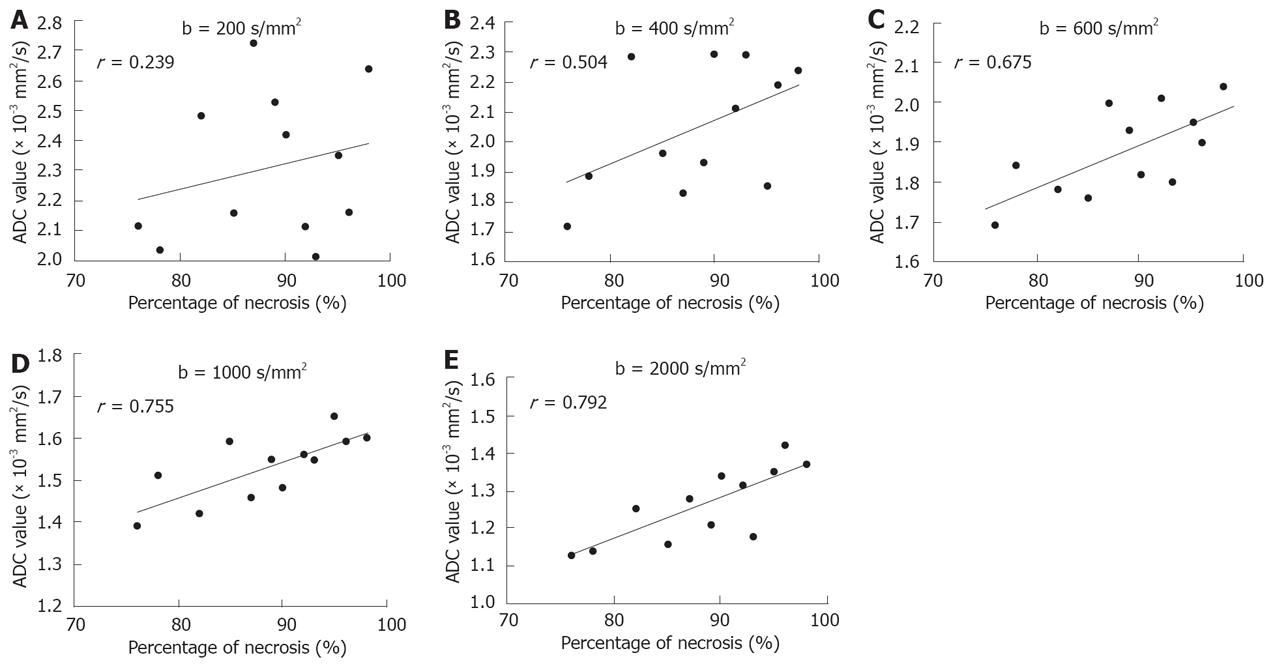
RESULTS: The quality of diffusion-weighted images diminished as the b value increased. A substantial decrease in the mean lesion-to-liver CNR was observed on both pre- and post-treatment DW images, the largest difference in CNR pre- and post-treatment was manifested at a b value of 1000 s/mm2 (P = 0.036 ). The effect of therapy on diffusion early after treatment was shown by a significant increase in ADCs (P = 0.007), especially with large b factors (≥ 600 s/mm2). The mean percentage of necrotic cells present within the tumor was 76.3%-97.5%. A significant positive correlation was found between ADC values and the extent of necrosis with all b values except for b200, a higher relative coefficient between ADC values and percentage of necrosis was found on DWI with b1000 and b2000 (P = 0.002 and 0.006, respectively).
CONCLUSION: An increasing b value of up to 600 s/mm2 would increase ADC contrast pre- and post-treatment, but decrease image quality. Taking into account both CNR and ADC measurement, diffusion-weighted imaging obtained with a b value of 1000 s/mm2 is recommended for monitoring early hepatic tumor response to TACE.
- Citation: Jiang ZX, Peng WJ, Li WT, Tang F, Liu SY, Qu XD, Wang JH, Lu HF. Effect of b value on monitoring therapeutic response by diffusion-weighted imaging. World J Gastroenterol 2008; 14(38): 5893-5899
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5893.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5893
| B value(s/mm2) | ADC(10-3 mm2/s) pre-treatment | ADC(10-3 mm2/s) post-treatment |
| 200 | 2.32 ± 0.53 | 2.41 ± 0.62 |
| 400 | 1.88 ± 0.45 | 1.99 ± 0.60 |
| 600 | 1.49 ± 0.26 | 1.79 ± 0.43 |
| 1000 | 1.19 ± 0.32 | 1.52 ± 0.42 |
| 2000 | 0.81 ± 0.29 | 1.22 ± 0.36 |
Transcatheter hepatic arterial chemoembolization (TACE) remains the initial treatment for unresectable hepatocellular carcinoma (HCC)[1]. Evaluating the therapeutic response of HCC to TACE is critical in assessing the success of treatment and deciding therapeutic plan. Diffusion-weighted imaging (DWI) enables noninvasive characterization of biologic tissues based on their water diffusion properties. It is theoretically possible to quantify the combined effects of capillary perfusion and water diffusion in vivo by an apparent diffusion coefficient (ADC)[2], and its value is equal to the true diffusion coefficient D when diffusion is the only type of motion. Diffusion-weighted images are obtained by acquiring T2-weighted images with the addition of diffusion weighting gradient known as the “b value”. Generally, the larger the b values used, the lower the ADC values owing to the contribution of perfusion. Large b factors should be chosen for more precise evaluation of ADC values of the tumor. However, image quality will be greatly diminished if large b factors are used. Thus, it is very important to select a suitable b value to evaluate the tumor response accurately. Diffusion-weighted imaging has recently been used to monitor tumor response after therapy. However, there are considerable discrepancies in the selection of b values in previous reports[3-7]. In our study, we compared different b-value DWI in evaluation of hepatic tumor necrosis after TACE in rabbits to explore the optimal b value.
Fifteen male adult New Zealand white rabbits including three carrier rabbits (Animal Laboratory, Fudan University) with an average weight of 2.5 kg, were used in this experiment. The Animal Care and Use Subcommittee at Fudan University approved this experimental procedure. VX2 carcinoma strain was maintained by successive transplantation into the hind limb of a carrier rabbit. Tumor cell suspension was implanted with one subcutaneous injection into the hind leg of each carrier rabbit and grown for 2 wk. All animals were intramuscularly anesthetized with a mixture of ketamine hydrochloride (0.1 g/kg) and diazepam (5 mg/kg). The tumor was surgically excised from the carrier rabbit and placed in normal saline. Tumor tissue was dissociated into approximately 1-2 mm3 fragments and suspended in 5 mL normal saline, then aspirated into a 1 mL injector. A midline laparotomy was performed to expose the liver of the recipient rabbit, and tumor suspension (0.1-0.2 mL) was implanted in the left lobe of liver via an 18-gauge needle. The abdomen was closed in two layers. The tumors were allowed to grow to 1-2 cm in diameter, which typically required 2 wk.
Conventional and diffusion-weighted imaging was performed before and 3 d after therapy using the same protocol.
TACE was performed under the guide of digital subtraction angiography (Infinix Vc-i, Toshiba, Japan). Animals were intramuscularly anesthetized with a mixture of ketamine hydrochloride (0.1 g/kg) and diazepam (5 mg/kg). Vascular access was achieved in the femoral artery through surgical cut down. Celiac angiography was performed to identify the hepatic arterial anatomy and the feeder artery of the tumor using a 3-F catheter (Cook, Bloomington, India). The left hepatic artery, which exclusively supplies blood flow to the tumor, was catheterized selectively. When the catheter was adequately positioned in the left hepatic artery after celiac arteriography was performed, a chemoembolization mixture consisting of 5 mg doxorubicin (adriamycin; Farmitalia Carlo Erba, Italy) and 1 mL ethiodized oil (lipiodol; Andre Guerbet, France) was injected carefully into the artery. Digital spot images were obtained after chemoembolization. The catheter was then removed, and the femoral artery was ligated.
MR scanning was performed on a 1.5 T superconducting magnet (Signa Twinspeed excite, GE Medical Systems, USA) equipped with a maximum gradient strength of 40 mT/m. All images were acquired using a phased array knee coil. In all rabbits, unenhanced and contrast-enhanced T1-, T2-, and diffusion-weighted images were obtained in the axial, and/or coronal, sagittal plane, respectively. Diffusion-weighted images were obtained before contrast medium injection. The rabbits were anesthetized with a combination of ketamine hydrochloride and diazepam as described above. Each animal was placed in the knee coil at supine position with its abdomen fastened using a belt to control the motion artifacts caused by breathing.
T2-weighted fast spin-echo images (TR/TE, 2800/72.4; matrix, 512 × 512), and T1-weighted spin-echo images (TR/TE, 350/9.0; matrix, 256 × 256) were obtained. DWI was performed in the axial plane using a single-shot echo planar imaging (EPI) sequence with the following parameters: TR/TE = 3000-4000 /50.9-70.2 ms, FOV = 12 cm × 12 cm, pixel matrix = 256 × 256, section thickness = 3 mm, intersection gap= 0.5 mm. Different values of b factor (0, 200, 400, 600 or 1000, 2000 s/mm2) were used. Then, 0.05 mmol/kg gadopentetate dimeglumine (Magnevist; Schering, Germany) was administered intravenously, and fat-saturated spoiled gradient-echo T1W sequences were obtained. Therefore, the overall scan time was approximately 15 min.
ADC maps were automatically generated on the post processing workstation (ADW4.0, GE Medical Systems, USA). One experienced radiologist established ROIs in the tumors, lesion-free liver parenchyma, and background on all series of diffusion-weighted images. For heterogeneous tumors, regions of interest (ROIs) covered the entire tumor at the maximum section consisting of at least 100 pixels, and then were copied to the corresponding ADC maps, from which the ADC value with different b factors for each ROI was calculated. Each of the signal intensities (SI) and ADCs was measured three times, and the measurements were averaged. CNR performed on all DWI series was calculated using the following formula: CNR = (SIlesion-SIliver)/SDnoise, where SIlesion and SIliver are the signal intensity of the tumor and liver, respectively, and SD is the standard deviation from the background noise.
All animals were sacrificed by giving an intravenous pentobarbital overdose immediately after the completion of MR imaging. The tumors were surgically removed and fixed in a 10% formaldehyde solution. From each tumor three 5 μm thick sections corresponding to the image planes were cut and stained with hematoxylin and eosin (H&E) and analyzed under a light microscope. Viable tumor and tumor necrosis were identified on these sections and correlated to the corresponding spin-echo images and the ADC maps. The percentage of necrotic area in each tumor was then calculated by an experienced pathologist.
Statistical analysis was performed using SPSS 13.0. ADC values were presented as mean ± standard deviation (SD). Pearson’s correlation test was used to test for the relationship between ADC values and extent of necrosis. Differences in the ADCs pre- and post-treatment were assessed with the paired Student’s t-test. The correlation between ADC, CNR and b factors was analyzed. P < 0.05 was considered statistically significant.
TACE was performed successfully in all animals, and no animals died within 3 d after the procedure. The left hepatic artery exclusively supplied blood flow to the tumor. A region of hypervascular blush was noted on the left side of the upper abdomen. Selective accumulation of deposits of iodized oil was observed in the hepatic tumors at digital spot images performed immediately after TACE.
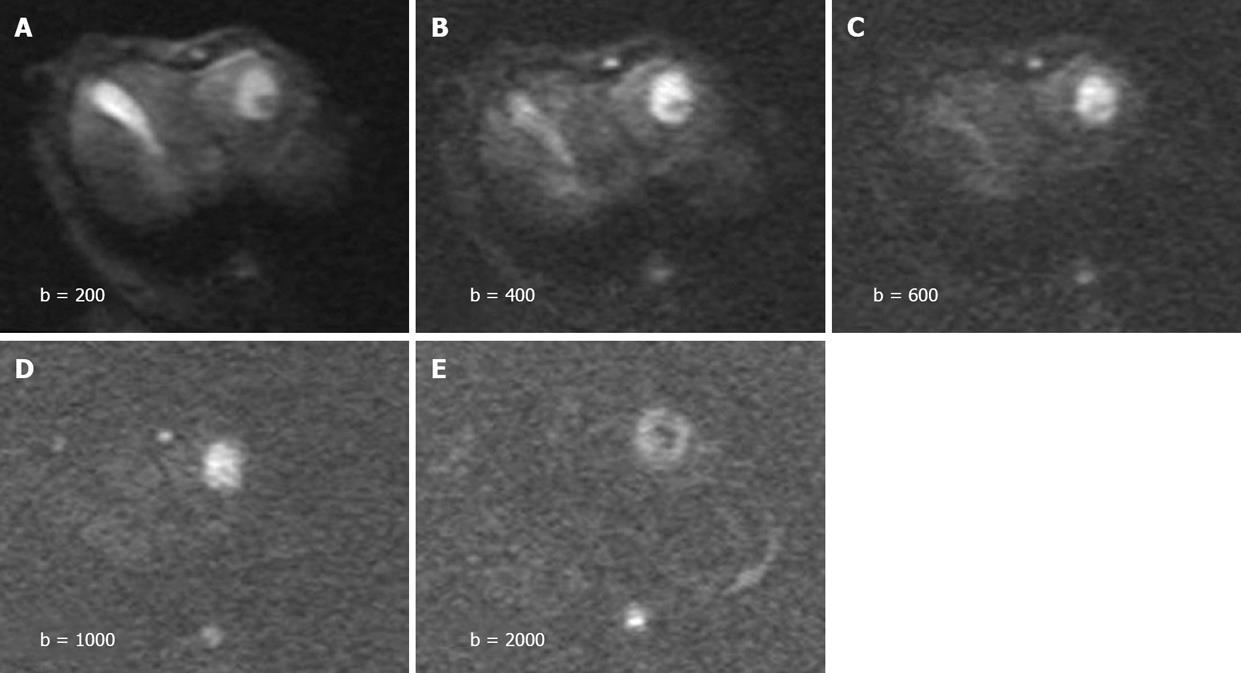
The tumors were slightly inhomogeneous hyperintense on T2-weighted images, hypointense on T1-weighted images. A marked peripheral enhancement pattern was noted on contrast-enhanced T1WI before and after TACE, with centrally non-enhancing regions corresponding to the necrotic area. However, the margin between the viable tumor and its surrounding liver parenchyma was inconspicuous. On DWI obtained before treatment with a b value of 200 or 400 s/mm2, both the tumor and gallbladder presented with hyperintense signals (Figure 1). On DWI with a b value equal to or larger than 600 s/mm2, the gallbladder was depicted as a region of hypointense signal attenuation, whereas the tumor remained hyperintense (Figure 1). On DWI obtained after treatment, the viable tumor presented with hyperintense signals indicating a restricted diffusion capacity, whereas a necrotic area was depicted as hypointense indicating free diffusion. The image quality diminished greatly with increasing b value especially on b2000 DWI (Figure 1). A substantial decrease in the mean lesion-to-liver CNR was observed on both pre- and post-treatment DW images. On these series of DW images, the largest difference in CNR pre- and post-treatment was manifested at a b value of 1000 s/mm2 (P = 0.036, Figure 2).
The early effect of TACE on diffusion was shown by a substantial increase in ADC (P = 0.007), especially with large b factors (≥ 600 s/mm2, Figure 3). These data are summarized in Table 1.
At microscopic examination, tumors treated with TACE revealed massive necrosis involving both the peripheral and central regions of the tumor. The mean percentage of necrotic cells present within the tumor was 76.3%-97.5% (mean 89.1%). A significant positive correlation was found between ADC values and extent of necrosis with all b values except for b200. A higher relative coefficient between ADC values and percentage of necrosis was found on DWI with b1000 and b2000 (P = 0.002 and 0.006, Figure 4).
Total necrosis after TACE has been reported to be as low as 10%-20%[8,9], and the presence of residual or recurrent tumor is inevitable. Precise evaluation with imaging modalities at an early stage is important to determine whether the tumor needs further treatment. The tumor volume has generally been used as an indicator of therapeutic response. However, necrotic tumors do not shrink until 1-2 mo after chemoembolization[10]. Besides, the apparent post-treatment increase may be due to the visualization of surrounding edematous changes. Thus, changes in tumor volume fail to predict the histological tumor response.
Diffusion-weighted imaging provides insights into tumor behavior for monitoring treatment response, thus enabling noninvasive depiction of molecular diffusion which is the Brownian motion of water protons in biologic tissues. Calculation of the ADC allows quantification of that motion[11-16]. The sensitivity of diffusion-weighted imaging to water motion can be varied by changing b value, which is a function of diffusion gradient strength, duration of the gradient, and interval between diffusion gradients. Diffusion-weighted imaging has been used to predict and monitor the effect of several treatment options and to differentiate between viable and necrotic tumor tissues[3-7]. In these studies, a variety of b values ranging from 0 s/mm2 to 4000 s/mm2 were used[3-7]. To our knowledge, however, comparison between DW images obtained with different b values on evaluation of the efficacy of TACE has not been reported.
In this study, we treated rabbits bearing VX2 tumors with TACE, whose vascularization is similar to that of human liver tumors[17-19]. Subsequently, the early tumor response to TACE was assessed via different b values on DWI to determine which b value is most suitable for evaluation.
In the present study, the tumor and gallbladder presented with hyperintense signals on DWI obtained with a low b value of 200 or 400 s/mm2. When the b value increased to 600 s/mm2, the gallbladder was depicted as a region of hypointense signal attenuation whereas the tumor remained hyperintense (Figure 1). Visual assessment of signal attenuation on DWI has been applied in tumor detection and characterization, however, signal intensity observed on DWI depends on both water diffusion and T2 relaxation time[20]. The relative contribution of T2 signal intensity to DWI, namely “T2 shine-through” effect, is a source of error in image interpretation. Tissues of organs with a long T2 relaxation time, such as gallbladder or cystic lesions, may appear hyperintense on DWI because of the T2 shine-through effect. This effect can be reduced by increasing the b value, but cannot be easily avoided. The ADC value is independent of magnetic field strength and can overcome the effects of T2 shine-through, thus allowing a more meaningful evaluation of tumor response to therapy.
In our study, the ADC values corresponded to the histopathologic rate of necrosis within the tumors, suggesting that diffusion-weighted imaging has a potential for early detection of tumor necrosis after TACE, which is in agreement with the findings in theoretical diffusion models and in vitro and in vivo studies[3,5-7,11]. DWI can differentiate viable and necrotic tissues by calculating ADC values because in the former, cell and intracellular membranes are intact, restricting molecular diffusion into viable tumors. Conversely, necrotic tumors are characterized by a breakdown of these membranes, thereby allowing free diffusion and an increase of diffusing molecules, resulting in an increased ADC value[11,14].
Comparison between different b-values on DWI revealed that the ADC values decreased with the increasing b value, and the difference in ADC values pre- and post-treatment was significant. A higher relative coefficient between ADC values and percentage of necrosis was found on DWI with b1000 and b2000, indicating that a high b-value on DWI is more sensitive in early detection of tumor necrosis. The signal intensity on diffusion-weighted images is a mixture of diffusion and perfusion. On DWI obtained with a low b-value, perfusion effects usually cause larger signal attenuation than diffusion effects[21,22]. In the presence of perfusion, the ADC value calculated from images with a low b value would be overestimated due to this additional cause of signal attenuation. At a high b value, ADC measurement will be relatively perfusion insensitive and theoretically more reflective of tissue cellularity and the integrity of cellular membranes[23,24]. A few studies on evaluation of tumor response to therapy with high b values on DWI have recently been reported[25,26]. Mardor et al[25] reported that a high-b-value on DWI is highly correlated to radiotherapy-treated human brain tumors. Roth et al[26] demonstrated that high-b-value diffusion-weighted MR imaging can be potentially used in early detection of response to chemotherapy.
However, in the present study, the image quality diminished with the increasing b value especially at b2000 on DWI, CNR measurements showed that b1000 on DWI increased the contrast pre- and post-treatment, suggesting that higher b values may increase the diffusion sensitivity by diminishing the T2 shine-through effect. High b values may also decrease the absolute difference in signal intensity between tumor and liver parenchyma. The results of our study suggest that an intermediate b value (i.e., 1000 s/mm2) may provide optimal visualization. In terms of the accuracy of ADC measurement, because the single-shot echo planar pulse sequence is very sensitive to magnetic susceptibility, resulting in geometric distortion artifacts that tend to be more severe when the b value is 2000 s/mm2, image distortion may cause significant errors in the measurement of ADC values.
In abdominal diffusion-weighted imaging, the most challenging technical difficulty is to overcome the effects of breathing motion, while retaining the sensitivity to the microscopic motion. To reduce the artifact caused by breathing, several attempts were made in our study. First, fast imaging acquisition was applied. The most common form of data acquisition is a single-shot read out, in particular single-shot echo planar imaging[27-30], since the acquired phase error due to bulk motion is equal in each phase encoding step and therefore does not affect the image reconstruction. With EPI, the fastest MR imaging technique, an image can be acquired within 50 ms and physiologic motion can be literally frozen-out, so it has been widely applied in DW imaging.
Second, respiratory rate should be controlled efficiently. To minimize the potential confounding artifacts caused by motion, Geschwind et al[13] acquired images shortly after the death of animals. We modified our protocol by fastening the abdomen of rabbits with a belt and using combined deep anesthesia. By this means, we obtained high quality images without virtually visible motion artifacts. The whole acquisition time was 15 min, and no animals died during the MR scanning procedure.
Our study had two major limitations. First, we placed the ROI covering the entire tumor. The ADC values of viable and necrotic area were not calculated. Second, due to the small population of animals, the results were not analyzed according to the tumor response or disease progress.
In summary, DWI can be used to assess the degree of tumor necrosis based on significant differences in ADC values. Based on the maximal contrast and changes in ADC values pre- and post-treatment, b values above 600 s/mm2 are recommended. We hold that the optimal b value should be 1000 s/mm2.
Necrotic tumor remnants or inflammatory fibrosis may not be accurately differentiated from residual tumor by conventional MR imaging early after therapy. DWI enables noninvasive characterization of biologic tissues based on their water diffusion properties and could provide information that is not readily available from conventional MR imaging. The sensitivity of diffusion-weighted imaging to water motion can be varied by changing the b value, which is a function of diffusion gradient strength. Thus, it is very important to select a suitable b value to evaluate the tumor response accurately.
Diffusion-weighted imaging has been used to predict and monitor the effect of several treatment options and to differentiate between viable and necrotic tumor tissues. A few studies on evaluation of tumor response to therapy using at high b values on diffusion-weighted imaging (DWI) have recently been reported. Mardor et al reported that a high-b-value on DWI has a higher correlation to radiotherapy-treated human brain tumors. Roth et al demonstrated that a high-b-value on diffusion-weighted MR imaging can be potentially used in detecting early response of tumors to chemotherapy in an animal model.
A major limitation of previous studies is the lack of comparison between DW images obtained with different b values. In this study, we treated rabbits bearing VX2 tumors with transarterial chemoembolization (TACE). Subsequently, the early response of tumor to TACE was assessed via different b values on DWI to determine which b value is most suitable for evaluation.
DWI can be used to assess the degree of tumor necrosis based on significant differences in the apparent diffusion coefficient (ADC) values. With regard to maximal contrast, changes in ADC values pre- and post-treatment, b values above 600 s/mm2 are recommended. Physicians should be aware of the trade-off between increased image distortion and ADC difference at higher b values, the optimal b value should be 1000 s/mm2.
DWI exploits the random, translational motion of water protons in biologic tissues, which causes phase dispersion of the spins resulting in signal loss. This signal loss can be quantified by calculating the ADC value, which refers to the specific diffusion capacity of a biologic tissue. The sensitivity of diffusion-weighted imaging to water motion can be varied by changing the b value, which is a function of diffusion gradient strength.
In the study, the authors tried to find the diffusion gradient b-factor that optimizes ADC measurement and contrast-to-noise (CNR) for assessing tumor response to TACE in a rabbit model. The image modalities and technique they used in this study were advanced both at home and abroad. The study design is perfect. The conclusion about the optimal b value for monitoring early hepatic tumor response to TACE is of clinical importance.
Peer reviewer: Dr. Li-Qin Zhao, Department of Radiology, Beijing Friendship Hospital Affiliate of Capital University of Medical Science, 95 Yongan Road, Xuanwu Distric, Beijing 100050, China
S- Editor Zhong XY L- Editor Wang XL E- Editor Zhang WB
| 1. | Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211-S221. [Cited in This Article: ] |
| 2. | Le Bihan DJ. Differentiation of benign versus pathologic compression fractures with diffusion-weighted MR imaging: a closer step toward the "holy grail" of tissue characterization? Radiology. 1998;207:305-307. [Cited in This Article: ] |
| 3. | Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, Kitajima M, Hirai T, Yamashita Y, Mizuta H. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: Initial results. Eur Radiol. 2006;16:2637-2643. [Cited in This Article: ] |
| 4. | Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307-308. [Cited in This Article: ] |
| 5. | DeVries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D, Pfeiffer KP, Lukas P, Judmaier W. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958-965. [Cited in This Article: ] |
| 6. | Seierstad T, Folkvord S, Roe K, Flatmark K, Skretting A, Olsen DR. Early changes in apparent diffusion coefficient predict the quantitative antitumoral activity of capecitabine, oxaliplatin, and irradiation in HT29 xenografts in athymic nude mice. Neoplasia. 2007;9:392-400. [Cited in This Article: ] |
| 7. | Tomura N, Narita K, Izumi J, Suzuki A, Anbai A, Otani T, Sakuma I, Takahashi S, Mizoi K, Watarai J. Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: possible prediction of treatment response. J Comput Assist Tomogr. 2006;30:496-500. [Cited in This Article: ] |
| 8. | Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293-299. [Cited in This Article: ] |
| 9. | Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, Lee HS, Kim CY, Han MC. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182:709-713. [Cited in This Article: ] |
| 10. | Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678. [Cited in This Article: ] |
| 11. | Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43:828-836. [Cited in This Article: ] |
| 12. | Lang P, Wendland MF, Saeed M, Gindele A, Rosenau W, Mathur A, Gooding CA, Genant HK. Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology. 1998;206:227-235. [Cited in This Article: ] |
| 13. | Geschwind JF, Artemov D, Abraham S, Omdal D, Huncharek MS, McGee C, Arepally A, Lambert D, Venbrux AC, Lund GB. Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol. 2000;11:1245-1255. [Cited in This Article: ] |
| 14. | Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003;45:208-213. [Cited in This Article: ] |
| 15. | Thoeny HC, De Keyzer F, Vandecaveye V, Chen F, Sun X, Bosmans H, Hermans R, Verbeken EK, Boesch C, Marchal G. Effect of vascular targeting agent in rat tumor model: dynamic contrast-enhanced versus diffusion-weighted MR imaging. Radiology. 2005;237:492-499. [Cited in This Article: ] |
| 16. | Baur A, Huber A, Arbogast S, Durr HR, Zysk S, Wendtner C, Deimling M, Reiser M. Diffusion-weighted imaging of tumor recurrencies and posttherapeutical soft-tissue changes in humans. Eur Radiol. 2001;11:828-833. [Cited in This Article: ] |
| 17. | Zhao JG, Feng GS, Kong XQ, Li X, Li MH, Cheng YS. Changes of tumor microcirculation after transcatheter arterial chemoembolization: first pass perfusion MR imaging and Chinese ink casting in a rabbit model. World J Gastroenterol. 2004;10:1415-1420. [Cited in This Article: ] |
| 18. | Kuszyk BS, Boitnott JK, Choti MA, Bluemke DA, Sheth S, Magee CA, Horton KM, Eng J, Fishman EK. Local tumor recurrence following hepatic cryoablation: radiologic-histopathologic correlation in a rabbit model. Radiology. 2000;217:477-486. [Cited in This Article: ] |
| 19. | Quan XY, Xie W, Zhang XL, Sun XJ, Zhu XL, Yan Z, Liang W. [Modification of the method for preparing rabbit liver VX2 tumor model and its MRI findings]. Nanfang Yike Daxue Xuebao. 2006;26:747-749. [Cited in This Article: ] |
| 20. | Provenzale JM, Engelter ST, Petrella JR, Smith JS, MacFall JR. Use of MR exponential diffusion-weighted images to eradicate T2 "shine-through" effect. AJR Am J Roentgenol. 1999;172:537-539. [Cited in This Article: ] |
| 21. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [Cited in This Article: ] |
| 22. | Morvan D. In vivo measurement of diffusion and pseudo-diffusion in skeletal muscle at rest and after exercise. Magn Reson Imaging. 1995;13:193-199. [Cited in This Article: ] |
| 23. | Thoeny HC, De Keyzer F, Boesch C, Hermans R. Diffusion-weighted imaging of the parotid gland: Influence of the choice of b-values on the apparent diffusion coefficient value. J Magn Reson Imaging. 2004;20:786-790. [Cited in This Article: ] |
| 24. | Wheeler-Kingshott CA, Thomas DL, Lythgoe MF, Guilfoyle D, Williams SR, Doran SJ. Burst excitation for quantitative diffusion imaging with multiple b-values. Magn Reson Med. 2000;44:737-745. [Cited in This Article: ] |
| 25. | Mardor Y, Pfeffer R, Spiegelmann R, Roth Y, Maier SE, Nissim O, Berger R, Glicksman A, Baram J, Orenstein A. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21:1094-1100. [Cited in This Article: ] |
| 26. | Roth Y, Tichler T, Kostenich G, Ruiz-Cabello J, Maier SE, Cohen JS, Orenstein A, Mardor Y. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004;232:685-692. [Cited in This Article: ] |
| 27. | Manenti G, Squillaci E, Di Roma M, Carlani M, Mancino S, Simonetti G. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissue using thin-slice echo-planar imaging. Radiol Med (Torino). 2006;111:1124-1133. [Cited in This Article: ] |
| 28. | Abdel Razek AA, Kandeel AY, Soliman N, El-shenshawy HM, Kamel Y, Nada N, Denewar A. Role of diffusion-weighted echo-planar MR imaging in differentiation of residual or recurrent head and neck tumors and posttreatment changes. AJNR Am J Neuroradiol. 2007;28:1146-1152. [Cited in This Article: ] |
| 29. | Koh DM, Scurr E, Collins DJ, Pirgon A, Kanber B, Karanjia N, Brown G, Leach MO, Husband JE. Colorectal hepatic metastases: quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006;16:1898-1905. [Cited in This Article: ] |