Published online Sep 14, 2008. doi: 10.3748/wjg.14.5306
Revised: August 18, 2008
Accepted: August 25, 2008
Published online: September 14, 2008
AIM: To determine if the presence H pylori or its virulence affect toll-like receptor 4 (TLR4) and TLR5 mRNA expression levels.
METHODS: For the in vivo assays, gastric biopsies were obtained from 40 patients and H pylori status was determined. For the in vitro assays, human gastric adenocarcinoma mucosal cells (AGS) were cultured in the presence or absence of twelve selected H pylori strains. H pylori strains isolated from culture-positive patients and selected strains were genotyped for cagA and vacA. The cDNA was obtained from mRNA extracted from biopsies and from infected AGS cells. TLR4 and TLR5 mRNA levels were examined by real-time PCR.
RESULTS: The presence of H pylori did not affect the mRNA levels of TLR4 or TLR5 in gastric biopsies. The mRNA levels of both receptors were not influenced by the vacA status (P > 0.05 for both receptors) and there were no differences in TLR4 or TLR5 mRNA levels among the different clinical presentations/histological findings (P > 0.05). In the in vitro assay, the mRNA levels of TLR4 or TLR5 in AGS cells were not influenced by the vacAs1 status or the clinical condition associated with the strains (P > 0.05 for both TLR4 and TLR5).
CONCLUSION: The results of this study show that the mRNA levels of TLR4 and TLR5 in gastric cells, both in vivo and in vitro, are independent of H pylori colonization and suggest that vacA may not be a significant player in the first step of innate immune recognition mediated by TLR4 or TLR5.
-
Citation: Garza-González E, Bocanegra-García V, Bosques-Padilla FJ, Flores-Gutiérrez JP, Moreno F, Perez-Perez GI. mRNA levels of TLR4 and TLR5 are independent of
H pylori . World J Gastroenterol 2008; 14(34): 5306-5310 - URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5306.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5306
| H pylori genotype (n = 20 strains) | |||
| Diagnosis | vacA s1 | vacA s2 | cagA |
| Gastritis | 5/15 | 10/15 | 15/15 |
| Intestinal metaplasia | 4/4 | 0/4 | 4/4 |
| Antral ulcer | 0/1 | 1/1 | 1/1 |
| Atrophic gastritis | 0/0 | 0/0 | 0/0 |
H pylori is a Gram-negative, flagellated bacterium that colonizes the gastric mucosa of approximately two-thirds of the world’s population and is the primary cause of peptic ulcers and gastric adenocarcinoma. The complex interactions between different H pylori strains, the host immune system or environmental factors (or combinations thereof) are responsible for the significant variability in disease presentation associated with H pylori infection[1].
Vacuolating cytotoxin (VacA) and the CagA protein are the two major virulence markers usually associated with H pylori pathogenicity. VacA induces the formation of intracellular vacuoles in epithelial cell lines. Aside from its direct cell-damaging effect in vitro, VacA also plays a major role in inducing cytoskeletal changes, apoptosis and suppression of epithelial cell proliferation[2]. The vacA gene is present in all H pylori strains and contains at least two variable domains. The s-region, which encodes the signal peptide, exists as either an s1 or s2 isoform. The vacA s1 genotype has been linked to increased disease severity[2,3].
Some H pylori strains contain a pathogenicity island, which carries a number of virulence factors, including cagA, which is considered to be a marker for this island. A type IV secretory system translocates the CagA protein into host epithelial cells where it is phosphorylated by host-cell kinases[4]. H pylori cagA-positive strains have been associated with more severe inflammation of the gastric mucosa and more severe disease manifestations[1,2].
Toll-like receptors (TLRs) are a family of mammalian homologs of the Drosophila Toll proteins and, in mammalian systems, TLR4 confers responsiveness to Gram-negative lipopolysaccharide (LPS), while TLR5 recognizes flagellin[5]. Previous studies have shown that gastric epithelia express both TLR4 and TLR5[6-9]. Here, we studied the mRNA levels of TLR4 and TLR5 in gastric epithelial cells to determine if distinctive changes in the levels of mRNA could be affected by the presence of toxigenic and non-toxigenic (in particular vacA+ strains) H pylori strains.
Forty patients (mean age, 58.3 years; age range, 18-81 years; F/M, 25/15) with indications of upper gastroduodenal endoscopy were included and the study was approved by the local ethics committee. Eighteen biopsy specimens were obtained from each patient. Total RNA was extracted from four of the biopsies from each patient (two from the antrum and two from the corpus) using Trizol® reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. Eight biopsies were used for histological evaluation; two from the lesser curvature, two from the greater curvature, two from the incisura angularis, and two from the prepyloric region. Biopsies were fixed, paraffin embedded and examined histologically after hematoxylin-eosin staining.
Patients’H pylori status was determined by histology, the rapid urease test performed by an in-house validated test on two biopsies (one from the antrum and one from the corpus)[10], and culture analysis performed by standard methods on four biopsies (two from the antrum and two from the corpus)[11]. Bacterial genomic DNA was extracted from H pylori strains and typing for cagA and vacA was performed using primers previously described[3,12]. Patients were considered H pylori-positive when at least two of the diagnostic tests were positive.
From our collection, we selected 12 strains that were isolated from patients with gastritis, distal gastric cancer, and peptic ulcer disease (4 from each pathologic/histologic finding). All strains were genotyped for vacA and cagA as described above. Human gastric epithelial AGS CRL-1739 cells were grown in RPMI 1640 supplemented with 10% FBS. H pylori bacteria were added at a multiplicity of infection (MOI) of 100:1, followed by a phosphate buffered saline (PBS) pH 7.4 wash to remove non-adherent bacteria. After 24 h, total RNA was isolated using the RNA tissue kit (Gentra Systems, Minneapolis, MN). The H pylori J99 ATCC 700824 strain was used as a control in all experiments.
Five micrograms of RNA were reverse-transcribed using SuperScript III (Invitrogen) in a 20 μL reaction volume using oligo dT primers. The resulting cDNA was real-time-amplified in a final volume of 25 μL. The mix contained 1 U of Hot-Start Taq DNA polymerase (Invitrogen), 1 × reaction buffer, 200 μmol/L of each deoxynucleoside triphosphate (dNTPs), 3 mmol/L MgCl2, 0.3 μmol/L of each primer and 0.25 × SYBR Green (Molecular Probes, Eugene OR). PCR was performed in glass capillaries using a Light Cycler instrument (Roche Applied Science, Indianapolis, IN). All reactions were performed in duplicate, and the thermal cycling conditions were 1 min at 94°C, followed by 35 cycles of 94°C for 10 s, 59°C for 10 s and 72°C for 10 s with a ramp of 5°C/s. Real time PCR for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed as described previously[7]. The relative amounts of the PCR products were calculated as the ratio of each TLR mRNA to GAPDH mRNA.
Comparisons were performed using a non-paired Student’s t test. Analyses were carried out using the Statistics software 7.0 (Melbourne, Australia).
The majority, 57.5 % (n = 23), of the patients examined were H pylori positive (F/M, 14/9; 22-81 years; 53.8 ± 20.3). Of the 40 patients in the study, 29 had gastritis confirmed by histology, 5 had intestinal metaplasia (IM) and 4 had antral ulcers. Only two patients had atrophic gastritis. All of the H pylori strains that were isolated were cagA-positive (Table 1).
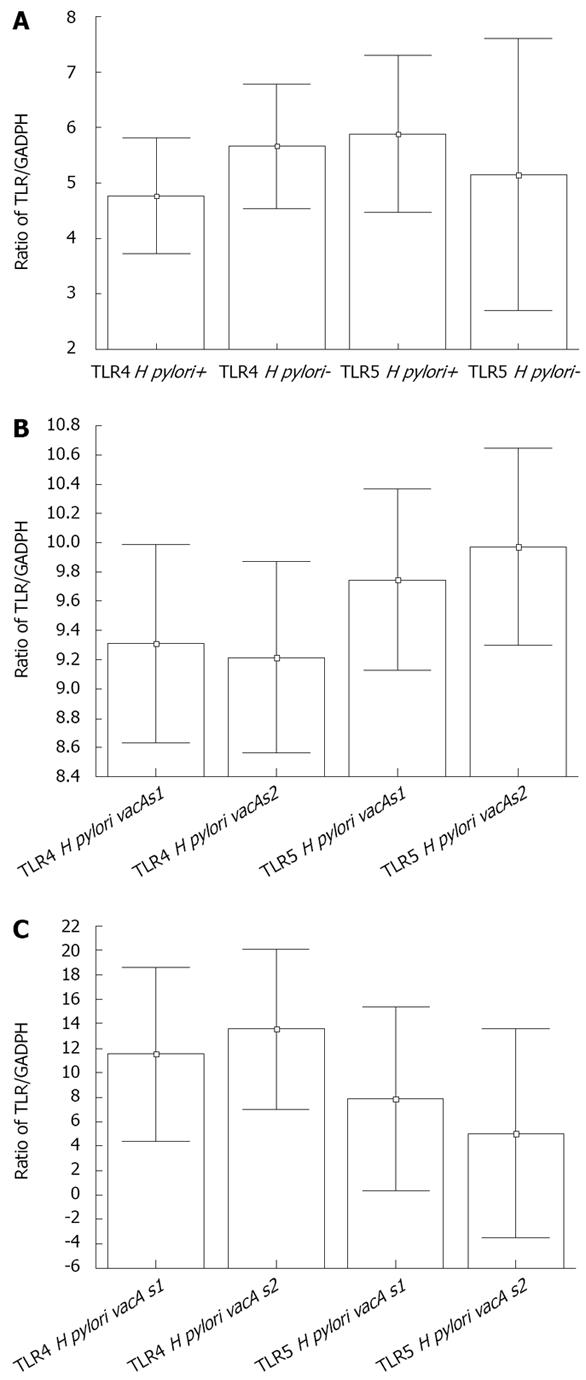
We plotted the TLR/GADPH ratios for both TLR4 and TLR5 in both infected and non-infected patients (Figure 1A). The presence of H pylori did not affect the mRNA levels of either Toll receptor. We did a similar analysis comparing patients infected with either H pylori vacAs1 or H pylori vacAs2 (Figure 1B). These data demonstrated that the mRNA levels of TLR4 and TLR5 were not influenced by the vacA status (P > 0.05 for both receptors). There were no differences in TLR4 or TLR5 mRNA levels between patients with different clinical presentations/histological findings (P > 0.05, data not shown). We were not able extract conclusions with respect to cagA, because all strains were cagA positive.
Eleven (91.7%) of the selected strains were cagA-positive and there was an equal vacA s1 and s2 distribution between the strains examined. There were no differences in the mRNA levels of TLR4 or TLR5 regarding the vacAs1 status or the clinical condition associated with the infecting strains (P > 0.05 for both TLR4 and TLR5, Figure 1C).
Several studies have addressed the mRNA levels and protein expression of TLR4 and TLR5 in gastrointestinal cells or in AGS cells[6-9,13]. In this study, we examined the mRNA levels of TLR4 and TLR5 in gastric epithelial cells (biopsies and AGS cells) in order to determine if significant changes in mRNA levels could be related to the presence of H pylori or differences between the virulence of the strains. Our examination of gastric biopsies from infected and non-infected patients showed that there were no quantitative differences in the mRNA levels of these receptors regardless of whether H pylori was present or of the patient’s H pylori vacA status.
Gastritis is thought to precede the development of IM, antral ulcer or atrophic gastritis[1,2]. In this study, we included biopsies from 40 patients and among them, 29 had gastritis, 5 had IM, 4 had antral ulcers, and 2 had atrophic gastritis. When we compared the mRNA levels of both receptors between patients with gastritis and with each clinical condition/histological findings, we were unable to identify differences, suggesting that mRNA levels for both receptors may not be influenced by the infection process, or at least not at the time points selected for analysis.
Our analysis of TLR4 and TLR5 mRNA levels in AGS cells in the presence or absence of H pylori showed that the amounts of TLR4 and TLR5 mRNA in human gastric epithelial cells were independent of H pylori vacA status. In the in vitro assay, the analysis showed no differences in the amounts of TLR4 and TLR5 mRNA linked to different clinical conditions related to the H pylori strains selected.
Our results do not exclude the possibility of differential expression between TLR4 and TLR5 receptors since mRNA levels do not accurately reflect protein expression. In this study, we used quantitative real-time PCR, which is the most commonly used technique for studying mRNA expression levels[14]. This technique has some advantages, such as accuracy, sensitivity and reproducibility. Also, it allows for high throughput analyses and can be performed on very small samples. However, some problems associated with this technique must be addressed, such as the effects of different amounts of starting material, especially when analyzing biopsies. To deal with this variation, an internal control (housekeeping gene) must be simultaneously amplified with the gene of interest for normalization purposes[15]. In this study, even though we included analysis of GADPH as an internal control we could not assume that protein expression levels of both receptors were equal based on the levels of detected mRNA.
An additional caveat was that the in vivo mRNA analysis of pooled biopsies from forty patients minimized the ability to identify biopsy-to-biopsy or patient-to-patient differences. Since RNA was extracted from all four antrum and corpus biopsies in the same vial, different gene expression profiles associated with different tissues would not have been detected. By analyzing four combined biopsies, it was possible that mRNA differences between corpus and antrum mucosal samples could have been missed. However, in the in vitro assay the mRNA levels obtained following infections with 12 different H pylori strains showed no differences in TLR expression. Although this is a uniform tissue, the observation that at least different H pylori strains did not affect TLR mRNA levels suggested that the same results would be observed in biopsy samples.
Some investigators have suggested that the subcellular distribution of receptors, rather than TLR expression level, could be relevant in the pathogenesis of inflammatory diseases because the expression of these receptors seems to be constitutive[7]. Our results are consistent with this view. Nonetheless, a study examining the cellular distribution of expression in relation to H pylori vacA status would be an interesting issue to address.
Schmausser et al[9], reported that gastric epithelium with intestinal metaplasia and dysplasia expressed TLR4 and TLR5. They demonstrated that 17 out of 22 patients strongly expressed TLR4 (77.27%) and all 22 patients with gastric carcinoma expressed TLR5. Our study confirmed the presence of TLR4 and TLR5 mRNA, which preceded the expression of both receptors.
It is likely that evaluating the roles of other Toll-like receptors would help elucidate differences in disease manifestation and severity of diseases caused by H pylori infections. Valuable information regarding the recognition of whole H pylori or its LPS by TLR2 has been reported. Smith et al[16] demonstrated that gastric epithelial cells recognized and responded to H pylori infection, at least in part, via TLR2 and that H pylori LPS was a TLR2 agonist.
Additionally, Mandell et al[17] demonstrated that cytokine responses to whole H pylori were mediated by TLR2. Based on these investigations, more work examining the role of TLR2 in relation to the H pylori vacA status is needed. The results of this study show that the TLR4 and TLR5 mRNA levels in gastric cells both in vivo and in vitro are independent of H pylori and suggest that vacA may not be involved in the first steps of innate immune-recognition of H pylori.
H pylori is the primary cause of peptic ulcers and gastric adenocarcinoma. The variability of clinical manifestations is associated with bacterial, host immune responses and environmental factors.
Gastric epithelia express toll-like receptor 4 (TLR4) and TLR5. We studied the mRNA levels of TLR4 and TLR5 in gastric epithelial cells to determine if distinctive changes in mRNA levels could be influenced by the presence of toxigenic H pylori strains.
In this study, we analyzed the mRNA levels of both TLR4 and TLR5 in gastric biopsies from infected and non-infected patients and in AGS cells infected with H pylori. We correlated these results with the vacA status of the strains. There were no quantitative differences in the mRNA levels of these receptors regardless of H pylori presence or the H pylori vacA status both in gastric biopsies and in AGS cells.
The mRNA levels of TLR4 and TLR5 in gastric cells both in vivo and in vitro are independent of H pylori or their vacA status.
The results show that the mRNA levels of TLR4 and TLR5 in gastric cells are not influenced by H pylori vacA status and suggest that vacA may not be a significant player in the first step of innate immune recognition mediated by TLR4 or TLR5. It seems innovative and very interesting.
Peer reviewer: Dr. Limas Kupcinskas, Professor, Gastro-enterology of Kaunas University of Medicine, Mickeviciaus 9, Kaunas LT 44307, Lithuania
S- Editor Zhong XY L- Editor Negro F E- Editor Ma WH
| 1. | Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767-773. [Cited in This Article: ] |
| 2. | Figueiredo C, Machado JC, Yamaoka Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2005;10 Suppl 1:14-20. [Cited in This Article: ] |
| 3. | Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. [Cited in This Article: ] |
| 4. | Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [Cited in This Article: ] |
| 5. | Ferrero RL. Innate immune recognition of the extracellular mucosal pathogen, Helicobacter pylori. Mol Immunol. 2005;42:879-885. [Cited in This Article: ] |
| 6. | Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496-3502. [Cited in This Article: ] |
| 7. | Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, Eck M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521-526. [Cited in This Article: ] |
| 8. | Backhed F, Rokbi B, Torstensson E, Zhao Y, Nilsson C, Seguin D, Normark S, Buchan AM, Richter-Dahlfors A. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J Infect Dis. 2003;187:829-836. [Cited in This Article: ] |
| 9. | Schmausser B, Andrulis M, Endrich S, Muller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179-185. [Cited in This Article: ] |
| 10. | Flores-Orta D, Bosques-Padilla F, Gomez-Leija G, Frederick F. Comparative study of rapid urease test (Hazell test) vs CLO-test in the diagnosis of Helicobacter pylori infection. Gut. 1997;41 Suppl 3:A160. [Cited in This Article: ] |
| 11. | Perez-Perez GI. Accurate diagnosis of Helicobacter pylori. Culture, including transport. Gastroenterol Clin North Am. 2000;29:879-884. [Cited in This Article: ] |
| 12. | Rugge M, Busatto G, Cassaro M, Shiao YH, Russo V, Leandro G, Avellini C, Fabiano A, Sidoni A, Covacci A. Patients younger than 40 years with gastric carcinoma: Helicobacter pylori genotype and associated gastritis phenotype. Cancer. 1999;85:2506-2511. [Cited in This Article: ] |
| 13. | Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496-3502. [Cited in This Article: ] |
| 14. | Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155-166. [Cited in This Article: ] |
| 15. | Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169-193. [Cited in This Article: ] |
| 16. | Smith MF Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552-32560. [Cited in This Article: ] |
| 17. | Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446-6454. [Cited in This Article: ] |