Published online Jul 28, 2008. doi: 10.3748/wjg.14.4499
Revised: June 2, 2008
Accepted: June 9, 2008
Published online: July 28, 2008
AIM: To report our experience with computed tomography colonography (CTC) systematically performed in subjects with positive faecal occult blood test (FOBT) and an incomplete colonoscopy in the setting of a population-based screening for colorectal cancer (CRC).
METHODS: From April 2006 to April 2007, 43 290 individuals (age range 50-70) who adhered to the regional screening program for the prevention of CRC underwent immunochemical FOBT. FOBT was positive in 1882 subjects (4.3%). 1463 (77.7%) of these subjects underwent colonoscopy, 903 performed in a single center. Of 903 colonoscopies 65 (7.2%) were incomplete. Forty-two of these subjects underwent CTC. CTC was performed with a 16-MDCT scanner after standard bowel prep (polyethylene glycole) in both supine and prone position. Subjects whose CTC showed polyps or masses were referred to the endoscopist for repeat colonoscopy under sedation or underwent surgery. Per-lesion and per-segment positive predictive values (PPV) were calculated.
RESULTS: Twenty-one (50%) of 42 CTCs showed polyps or masses. Fifty-five of these subjects underwent a repeat colonoscopy, whereas 2 subjects underwent surgery for colonic masses of indeterminate nature. Four subjects refused further examinations. CTC correctly identified 2 colonic masses and 20 polyps. PPV for masses or polyps greater than 9 mm was of 87.5%. Per-lesion and per-segment PPV were, respectively, 83.3% and 83.3% for polyps greater or equal to 10 mm, and 77.8% and 85.7% for polyps of 6-9 mm.
CONCLUSION: In the context of a screening program for CRC based on FOBT, CTC shows high per-segment and per-lesion PPV for colonic masses and polyps greater than 9 mm. Therefore, CTC has the potential to become a useful technique for evaluation of the non visualized part of the colon after incomplete colonoscopy.
- Citation: Sali L, Falchini M, Bonanomi AG, Castiglione G, Ciatto S, Mantellini P, Mungai F, Menchi I, Villari N, Mascalchi M. CT colonography after incomplete colonoscopy in subjects with positive faecal occult blood test. World J Gastroenterol 2008; 14(28): 4499-4504
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4499.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4499
Randomized clinical trials have demonstrated that screening with faecal occult blood test (FOBT) reduces mortality for colorectal cancer (CRC)[1]. Accordingly, population-based screening with FOBT is currently recommended by the European community health associations and has been applied in many countries, including Italy[2].
Subjects with a positive FOBT are usually examined with a total colonoscopy which allows removal of polyps and histological diagnosis of the lesions. However, colonoscopy can be incomplete due to several reasons, including intolerance to the procedure, adhesions from previous surgery, redundant colon and the presence of stenosis. The reported rates of incomplete colonoscopy from various studies carried out in U.S. and Europe over the past 15 years range from 4% to 25%[34].
In order to complete evaluation of the colon, radiological examinations can be performed such as double contrast barium enema (DCBE)[5] and computed tomography colonography (CTC). In particular, several studies have shown that CTC is a valuable tool to evaluate the proximal colon after incomplete colonoscopy[6–10], and the American Gastroenterologists Association (AGA) recognized that CTC is indicated for adults with failed colonoscopy[11].
We report the results of CTC systematically performed in subjects with positive FOBT and incomplete colonoscopy in the context of a population-based screening programme for CRC with FOBT.
This prospective study was approved by our institutional review board; informed consent was obtained in all subjects. A population-based screening program for CRC has been active in the Tuscany Region, Italy, since 1998. The screening protocol is directed to all subjects aged 50-70 living in the regional area who are invited via mail every second year to perform immunochemical FOBT. Subjects with negative FOBT are notified of their result by mail and advised to repeat screening after two years. Subjects with positive test are invited to perform colonoscopy[12].
From April 2006 to April 2007, 43 290 asymptomatic individuals aged 50-70 years attended the FOBT-based Florence District screening program and were tested. FOBT was positive in 1882 (4.3%) subjects. These subjects were invited to undergo colonoscopy assessment: 1463 (77.7%) subjects underwent colonoscopy and 419 refused. 903 colonoscopies were performed in a single center by two experienced endoscopists.
According to the screening protocol, colonoscopy was performed without sedation. 838 (92.8%) colonoscopies were complete (i.e. the caecum was reached) and 65 (7.2%) were incomplete. The levels at which colonoscopy was interrupted were the sigmoid colon in 33 subjects, the descending colon in 21, the transverse colon in 8 and the ascending colon in 3. According to the endoscopist’s report, presumptive reasons for incomplete colonoscopy were dolichocolon (14.8%), diverticular disease (23.1%), adherences due to previous abdominal surgery (16.4%) or intolerance to the procedure (12.1%).
Forty-two of these 65 subjects (17 males, 25 females; mean age 60.7 years; age range 51-70) agreed to complete colonic examination with CTC and constitute of the base for this report.
CTC was performed within 6 wk after incomplete colonoscopy (mean interval 16 d). In those subjects in whom endoscopic polyp removal was performed during the incomplete colonoscopy, CTC was delayed for at least 1 mo after polypectomy.
All subjects underwent a standard bowel preparation for CTC with 4 L of a polyethylene glycole solution (Isocolan; Giuliani, Milan, Italy) administered the day before the procedure and a low residue diet for 3 d. All subjects received intravenously 30 mg of scopolamine butyl-bromide (Buscopan; Boehringer Ingelheim, Florence, Italy) before air insufflation, in order to improve colonic distension[13].
The subjects were placed on the right lateral decubitus and a 24 Fr rubber catheter, Foley type, with a small retention balloon (10 mL) was inserted into the rectum. After catheter positioning the patient was turned in supine position and colonic distension was obtained with manual insufflation of room air. Air was administered from an enema bag connected to the rectal tube with a maximum capacity of 2 L. Insufflation was performed by gently squeezing the enema bag during 3 to 5 min up to subject tolerance.
Both supine and prone CT scans were obtained in all subjects. Colonic distension was evaluated with an anterior-posterior scout view in both supine and prone position, and additional air was inflated using a manual bulb if distension was unsatisfactory. In one subject, unable to stay prone because of abdominal pain, a right lateral decubitus acquisition was obtained instead of the prone scan. Intravenous contrast medium was not used.
CTC was performed with a 16-MDCT scanner (Sensation 16; Siemens, Erlangen, Germany) using a detector configuration of 16 mm × 0.75 mm, 120 kVp, 50 effective mAs, tube rotation time of 500 ms and a pitch of 1.25. Data were reconstructed using a slice thickness of 1 mm with a reconstruction increment of 0.7 mm (30% overlap). For each acquisition CTDIvol was 4.15 mGy with a calculated equivalent dose of 3.5 mSv for females and 2.7 mSv for males (CT Patient Dosimetry Calculator, ImPACT; measures executed on MonteCarlo Phantom).
The images of each study were transferred to a workstation equipped with CTC dedicated software (Syngo; Siemens, Erlangen, Germany). The software provides axial, multi-planar reformatted (MPR), endoluminal surface-shaded images and double-contrast-like reconstructions of the colon. All studies were interpreted on the workstation by two readers, one experienced gastrointestinal radiologist and one radiology resident, by consensus.
Preliminarily, the degree of colonic distension was evaluated on axial images. The colon was divided into six segments: caecum, ascending, transverse, descending, sigmoid and rectum (the different segments were evaluated both in supine and prone acquisitions). Distension for each segment was graded on a scale from 0 to 3, in which a grade of 0 indicated complete collapse and a grade of 3 optimal distension[13]. The least-distended section of any individual segment was used to assign the overall distention score for that segment. Colonic distension was deemed clinically adequate if all segments had a score of 2 or 3 at least in supine or prone acquisition.
Moreover, we assessed the adequacy of preparation by evaluating the proportion of colonic segments containing residual faecal matter or fluid for each subject (no specific attempt was made to rank the amount of fluid or stool).
CTCs were evaluated with a primary 3D approach, using 2D for problem solving. In all cases, endoluminal navigation was performed from rectum to caecum and backwards for both supine and prone acquisitions. Then axial images were examined with an abdominal window (level 40 HU, width 350 HU), in order to discover areas of colonic wall thickening, and to look for extra-colonic findings.
All lesions detected at CTC were localized according to their segmental location in the colon. Each lesion was measured taking account of its maximum diameter on 2D images viewed with a bone window (level 400 HU, width 2000 HU).
All subjects with CTC showing polyps were referred to the endoscopist to repeat colonoscopy under sedation. Also subjects with colonic masses were referred to the endoscopist who evaluated in agreement with the subject the opportunity of a repeat colonoscopy or a surgical consult.
The results of repeat colonoscopy and/or the pathological findings on surgical specimens were used as a gold standard for CTC performance assessment. Lesions were measured by open biopsy forceps at endoscopy and with ruler for pathological specimens.
CTC findings were classified as true-positive or false-positive results. A true-positive lesion at CTC was defined as a lesion that was confirmed at repeat colonoscopy or at surgery, a lesion that was in the same or an adjacent colonic segment, and a lesion for which the size correlated within 50% of the diameter. A lesion was defined as false positive if the lesion reported at CTC was not detected at repeat colonoscopy, was not in the same or an adjacent colonic segment or there was more than a 50% discrepancy in the lesion diameter. Endoscopic evidence of polyps or masses at repeat colonoscopy not detected at CTC was assumed as a false negative result for CTC.
Descriptive statistics were used to calculate per-lesion and per-segment positive predictive values (PPV) for polyps equal or greater than 10 mm, for polyps of 6-9 mm and for smaller lesions (< 6 mm). In per-segment analysis the colon was divided into six segments (caecum, ascending, transverse, descending, sigmoid and rectum) and the segments examined by initial colonoscopies were excluded from the evaluation.
Subjects with a negative CTC did not undergo further examination and were scheduled for standard follow-up according to the screening protocol[12].
Complete colonic distension was obtained in 36 (85.7%) of 42 subjects. Considering both supine and prone acquisitions, the rectum in one patient and the sigmoid colon in 5 patients were not adequately distended. Incomplete distension was mainly due to advanced diverticular disease. The mean overall bowel distension scores were 2.75 ± 0.37 for supine position and 2.84 ± 0.23 for prone position. Either fluid and faecal residua, or inadequate distension precluded evaluation of 14 (5.6%) of 252 colonic segments [rectum (n = 1), sigmoid colon (n = 10), descending colon (n = 3)].
Twenty one (50%) of 42 CTCs showed polyps or colonic masses of indeterminate nature. Fifteen patients with polyps at CTC underwent repeat colonoscopy under sedation. Two patients with colonic masses of indeterminate nature were referred to surgical consult and underwent colectomy. Four patients with CTC findings of polyps smaller than 6 mm did not undergo repeat colonoscopy because of medical problems or refusal.
All repeat colonoscopic examinations were performed within a mean of 34 d after CTC (range 15 d to 6 mo) and were complete. No complications occurred after CTC or diagnostic and operative colonoscopy.
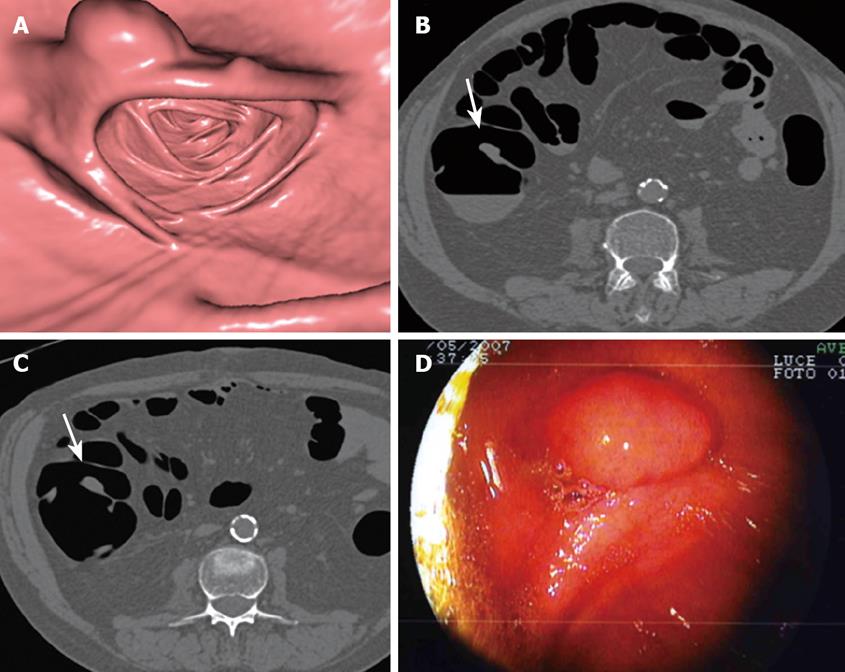
CTC correctly identified 20 polyps in segments not visualized at initial colonoscopy, and gave 7 false positive and 2 false negative results (Table 1). All polyps were endoscopically removed and histology was obtained (Figure 1). Of 20 polyps 11 were adenomas (2 tubulo-villous adenomas with high-grade dysplasia, 6 tubulo-villous adenomas, 3 tubular adenomas) and 9 hyperplastic or inflammatory polyps. No cancers were observed. The two false negative polyps not identified at CTC included one hyperplastic polyp and one tubular adenoma, both smaller than 6 mm. A total of 5 advanced adenomas were found at CTC and histologically proved.
| True positive | False positive | False negative | Per-lesion PPV (%) | |
| Polyps < 6 mm | 8 | 4 | 2 | 66.7 |
| Polyps 6-9 mm | 7 | 2 | 0 | 77.8 |
| Polyps ≥ 10 mm | 5 | 1 | 0 | 83.3 |
| Total | 20 | 7 | 2 |
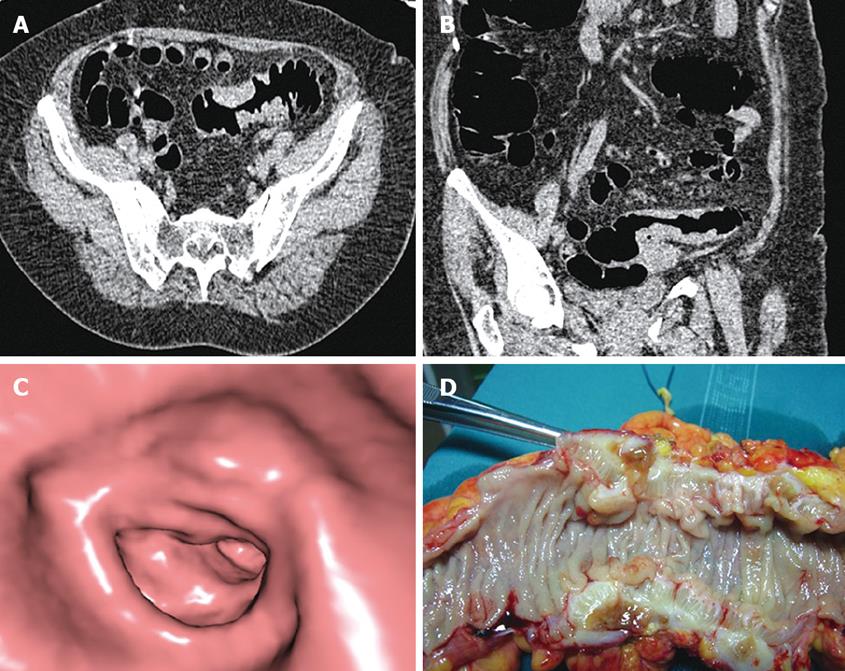
CTC correctly depicted two colonic masses of indeterminate nature at the level of the proximal sigmoid colon, which were found to be advanced diverticular disease complicated by stenosis at surgery (Figure 2). None of these patients had endoscopically visualized masses or adenomatous polyps at initial colonoscopy.
Per-segment analysis was performed on patients who completed repeat colonoscopy or underwent surgery (n = 17). On a total of 102 colonic segments, 26 segments examined by initial colonoscopies were excluded, and the analysis was based on the remaining 76 segments.
CTC showed a PPV for masses or polyps greater than 9 mm of 87.5%. Per-lesion and per-segment PPV were respectively 83.3% and 83.3% for polyps greater or equal to 10 mm, 77.8% and 85.7% for polyps of 6-9 mm, 66.7% and 50% for polyps smaller than 6 mm.
Diverticular disease was found in 20 subjects (47.6%) and 5 of these subjects (11.9%) showed signs of chronic diverticulitis such as diffuse wall thickening and pericolic fat stranding.
Major extra-colonic findings included aneurysm of the abdominal aorta (n = 1), renal masses (n = 2), hepatic focal lesion other than cystic (n = 1), splenomegaly (n = 1) and pulmonary nodules (n = 2).
Due to its natural history CRC is an ideal candidate for screening[14]. In fact, most CRC originate from pre-existing adenomatous polyps that, in 10 to 15 years, undergo malignant transformation[15]. Likelihood for malignant transformation is not the same for all adenomas. In particular adenomas equal or greater than 10 mm (advanced adenomas) tend to become malignant after an average of 5.5 years, whereas it is estimated that less than 1% of adenomas smaller than 10 mm contain a cancer[14]. Thus, advanced adenoma is a precancerous lesion and should be considered the main target of a screening test for CRC.
In the majority of screening programmes, subjects with positive FOBT are invited to undergo colonoscopy, which can be performed with or without sedation. Colonoscopies without sedation can be incomplete in up to 25% of the cases[4] and in our series incomplete colonoscopies were 7.2%. Before adoption of CTC in such cases, we previously performed DCBE.
Several studies showed that DCBE has a low accuracy in detecting colonic neoplasms, with sensitivity for adenomas greater than 9 mm in the range of 45%-50%[16]. CTC is more accurate in detecting colorectal neoplasms as shown in some meta-analyses in which the performance of CTC versus optical colonoscopy revealed sensitivity in the range of 85%-90% and specificity of about 95% for polyps greater than 9 mm[1718].
We evaluated the performance of CTC after incomplete colonoscopy in the setting of a large population based screening program with FOBT. In this context, CTC showed its potential for diagnostic assessment, identifying 2 colonic masses, 5 advanced adenomas and 6 smaller adenomas. CTC gave 7 false positive results which led to unnecessary repeat colonoscopy. One false positive was a polypoid lesion of 14 mm of the ascending colon that was visible only on the supine dataset. Four false positive results were for polyps smaller than 6 mm which should not be reported according to current recommendations[19].
The possibility that diverticular disease simulates with colonic masses is well known, as is the fact that the differential diagnosis with cancer can be difficult with CT[20]. In our series, CTC detected two colonic masses of the proximal sigmoid colon which showed CT features suspicious for malignancy, but were demonstrated by pathology to be due to diverticular disease in absence of any malignancy. In both cases, initial colonoscopy was interrupted at distal sigmoid colon because of advanced diverticular disease. In the two cases, the endoscopist and the subject decided not to perform a repeat colonoscopy and the subjects were referred to the surgeon to undergo colectomy.
Colonic distension and cleansing were adequate for an accurate examination in the majority of our cases. In some cases, colonic collapse or repletion by fluid or faeces precluded evaluation of the rectum, sigmoid or descending colon. This limitation can be partially overcome by the fact that lower colonic segments are usually examined at initial colonoscopy. Indeed, segments examined by initial colonoscopies were excluded from per-segment analysis.
Almost 50% of 42 patients of our study had diverticular disease which represented an obstacle to complete conventional colonoscopy. Our series showed that diverticular disease did not seriously compromise colonic distension and evaluation of the proximal colon at CTC.
Previous studies on CTC after incomplete colonoscopy have been conducted[6–10]. These studies were inhomogeneous regarding the patients’ selection, because they included asymptomatic as well as symptomatic subjects. Our results in terms of PPV, acquired in a selected group of screening subjects, were comparable with those obtained in the largest study on CTC after incomplete colonoscopy conducted by Copel et al[10]. They reported per-lesion PPV of 91.7% for masses, of 70% for polyps of 10 mm or greater, and of 30.4% for polyps of 6-9 mm[10]. In our small group of subjects, we considered, altogether masses and polyps greater than 9 mm obtaining a similar result (PPV of 87.5%). Our better results for medium sized polyps (6-9 mm) with a per-lesion PPV value of 77.8% might be due to thinner collimation for CT scanning and double reading of the examinations we utilize.
We observed a significant number of false positive results which led to unnecessary repeat colonoscopy. The use of faecal tagging should reduce the number of false positive, enabling a better distinction between polyps and faecal residues, as showed in a series of CTC after incomplete colonoscopies[9].
Our study had limitations. First, it was carried out on a small series of subjects. Second, repeat colonoscopy was conducted with segmental blinding, and this could have increased the number of false positive results of the CTC. Third, since subjects with negative CTC did not undergo further examinations, we could not evaluate sensitivity and specificity of CTC with respect to optical colonoscopy.
In conclusion, in the context of a population-based screening program for CRC based on FOBT, CTC showed a high per-segment and per-lesion PPV for colonic masses and polyps greater than 9 mm. Therefore, CTC has the potential to become a useful technique for evaluation of the non-visualized part of the colon after incomplete colonoscopy and should replace DCBE.
Colorectal cancer (CRC) is a relevant neoplastic disease for its high incidence and mortality. Due to its natural history CRC is suitable for screening. Screening with faecal occult blood test (FOBT) reduces mortality from CRC. Subjects with positive FOBT are usually examined by colonoscopy which can be incomplete.
Computed tomography colonography (CTC) is a non-invasive imaging technique with a high sensitivity and specificity in the diagnosis of colonic cancer and polyps equal or greater than 10 mm, which are the target for screening. Therefore, it might represent a second step examination before colonoscopy to examine subjects with positive FOBT.
This study on CTC after incomplete colonoscopy was conducted in the frame of a population based screening program. Previous reports on this topic were carried out in heterogeneous samples of symptomatic and asymptomatic subjects or patients with known colonic pathology.
In the context of a screening program with FOBT, CTC has high positive predictive value (PPV) for colonic masses or polyps equal or greater than 10 mm and should replace double contrast barium enema (DCBE) for evaluation of the non visualized part of the colon after incomplete colonoscopy.
CTC is a thin slice CT scan of the abdomen after adequate bowel preparation and colon insufflation in which data are reconstructed providing axial, multiplanar, and endoluminal views (virtual colonoscopy), in order to visualize colonic wall. Colonoscopy is the more accurate technique to evaluate colonic internal surface and it is performed passing a flexible tube with fiber optic through the anus. FOBT is a chemical test that can detect tiny traces of blood in the stool that may indicate the presence of CRC.
This paper shows the usefulness of CTC after insufficient colonoscopy in order to detect colorectal lesions. It was conducted as a part of population-based screening programme of CRC. It’s an interesting study.
| 1. | Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. |
| 2. | Recommendations on cancer screening in the European union. Advisory Committee on Cancer Prevention. Eur J Cancer. 2000;36:1473-1478. |
| 3. | Anderson ML, Heigh RI, McCoy GA, Parent K, Muhm JR, McKee GS, Eversman WG, Collins JM. Accuracy of assessment of the extent of examination by experienced colonoscopists. Gastrointest Endosc. 1992;38:560-563. |
| 4. | Marshall JB, Barthel JS. The frequency of total colonoscopy and terminal ileal intubation in the 1990s. Gastrointest Endosc. 1993;39:518-520. |
| 5. | Chong A, Shah JN, Levine MS, Rubesin SE, Laufer I, Ginsberg GG, Long WB, Kochman ML. Diagnostic yield of barium enema examination after incomplete colonoscopy. Radiology. 2002;223:620-624. |
| 6. | Macari M, Berman P, Dicker M, Milano A, Megibow AJ. Usefulness of CT colonography in patients with incomplete colonoscopy. AJR Am J Roentgenol. 1999;173:561-564. |
| 7. | Morrin MM, Kruskal JB, Farrell RJ, Goldberg SN, McGee JB, Raptopoulos V. Endoluminal CT colonography after an incomplete endoscopic colonoscopy. AJR Am J Roentgenol. 1999;172:913-918. |
| 8. | Neri E, Giusti P, Battolla L, Vagli P, Boraschi P, Lencioni R, Caramella D, Bartolozzi C. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology. 2002;223:615-619. |
| 9. | Gryspeerdt S, Lefere P, Herman M, Deman R, Rutgeerts L, Ghillebert G, Baert F, Baekelandt M, Van Holsbeeck B. CT colonography with fecal tagging after incomplete colonoscopy. Eur Radiol. 2005;15:1192-1202. |
| 10. | Copel L, Sosna J, Kruskal JB, Raptopoulos V, Farrell RJ, Morrin MM. CT colonography in 546 patients with incomplete colonoscopy. Radiology. 2007;244:471-478. |
| 11. | Rockey DC, Barish M, Brill JV, Cash BD, Fletcher JG, Sharma P, Wani S, Wiersema MJ, Peterson LE, Conte J. Standards for gastroenterologists for performing and interpreting diagnostic computed tomographic colonography. Gastroenterology. 2007;133:1005-1024. |
| 12. | Grazzini G, Castiglione G, Ciabattoni C, Franceschini F, Giorgi D, Gozzi S, Mantellini P, Lopane P, Perco M, Rubeca T. Colorectal cancer screening programme by faecal occult blood test in Tuscany: first round results. Eur J Cancer Prev. 2004;13:19-26. |
| 13. | Taylor SA, Halligan S, Goh V, Morley S, Bassett P, Atkin W, Bartram CI. Optimizing colonic distention for multi-detector row CT colonography: effect of hyoscine butylbromide and rectal balloon catheter. Radiology. 2003;229:99-108. |
| 14. | Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594-642. |
| 15. | Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251-2270. |
| 16. | Winawer SJ, Stewart ET, Zauber AG, Bond JH, Ansel H, Waye JD, Hall D, Hamlin JA, Schapiro M, O’Brien MJ. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med. 2000;342:1766-1772. |
| 17. | Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, Atkin W. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237:893-904. |
| 18. | Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635-650. |