Published online Jul 7, 2008. doi: 10.3748/wjg.14.4093
Revised: May 10, 2008
Accepted: May 17, 2008
Published online: July 7, 2008
Non-Hodgkin lymphoma is a rare cause of biliary obstruction. To the best of our knowledge, non-Hodgkin lymphoma in the peripancreatic region causing obstructive jaundice with simultaneous portal vein (PV) invasion has not yet been reported. We present a 50-year-old patient with obstructive jaundice whose extrahepatic portal vein was obstructed by the invasion of a peripancreatic non-Hodgkin lymphoma. The patient denied any other symptoms such as recurrent fever, night sweat and loss of body weight. Computed tomography (CT) revealed a 10 cm mass in the retroperitoneal space behind the head of the pancreas causing obstruction of the distal bile duct and the PV. A pylorus-preserving pancreaticoduodenectomy combined with a PV resection was performed. The PV was reconstructed using an autologous right internal jugular vein graft. The resected specimen showed endoluminal invasion of both the bile duct and the PV. Histological examination showed the mass consisting of diffuse sheets of large malignant lymphoid cells. These cells were positive for CD20 and CD79a, partially positive for CD10, and negative for CD3, CD4, CD5, CD8 and CD30. The pathologic diagnosis was diffuse large B-cell type non-Hodgkin lymphoma and the patient was transferred to the Department of Hematology and Oncology for chemotherapy. He received four cycles of combined chemotherapy including cyclophosphamide, doxorubicin, vincristine and prednisone plus rituximab, and three cycles of intrathecal chemoprophylaxis including methotrexate, cytosine arabinoside and prednisone. The patient is alive with no evidence of the disease for 7 mo after operation and will receive additional courses of chemotherapy.
- Citation: Hashimoto M, Umekita N, Noda K. Non-Hodgkin lymphoma as a cause of obstructive jaundice with simultaneous extrahepatic portal vein obstruction: A case report. World J Gastroenterol 2008; 14(25): 4093-4095
- URL: https://www.wjgnet.com/1007-9327/full/v14/i25/4093.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4093
Non-Hodgkin lymphoma is a rare cause of biliary obstruction[12]. In a retrospective study, biliary obstruction secondary to non-Hodgkin lymphoma was found in 3 (0.3%) of 1123 patients with malignant biliary obstruction, and obstructive jaundice was found in one of them[1]. On the other hand, vascular invasion due to non-Hodgkin lymphoma has been described only as case reports including a case of primary non-Hodgkin lymphoma of the liver with bile duct invasion and portal venous tumor thrombus[34].
To the best of our knowledge, non-Hodgkin lymphoma in the peripancreatic region causing obstructive jaundice with simultaneous portal vein (PV) invasion has not yet been reported. Here we present a patient with obstructive jaundice whose extrahepatic PV was obstructed by a peripancreatic non-Hodgkin lymphoma.
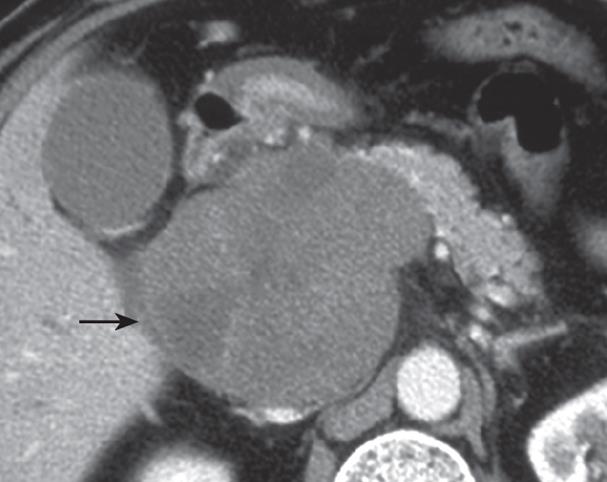
A 50-year-old man was reported to have an elevated serum lactate dehydrogenase (LDH) level (269 IU/L) by medical checkup in July 2007. He consulted with a physician in the following month. Serum LDH level continued to increase (313 IU/L). Abdominal ultrasonography was scheduled but he did not show up. On October 9, 2007, he presented with jaundice and was referred to our hospital on the next day. The patient denied any other symptoms such as recurrent fever, night sweat, and loss of body weight. His past history was unremarkable excluding a history of nephritis in his childhood. He had smoked 30 cigarettes per day, and was not alcoholic. His height was 173 cm and body weight 73 kg, blood pressure 124/79 mmHg and pulse rate 68 beats/min. No superficial lymphadenopathy was palpable. Computed tomography (CT) revealed a low-density mass, 10 cm in diameter, in the retroperitoneal space behind the pancreas head (Figure 1), due to which the distal bile duct and the PV were obstructed.
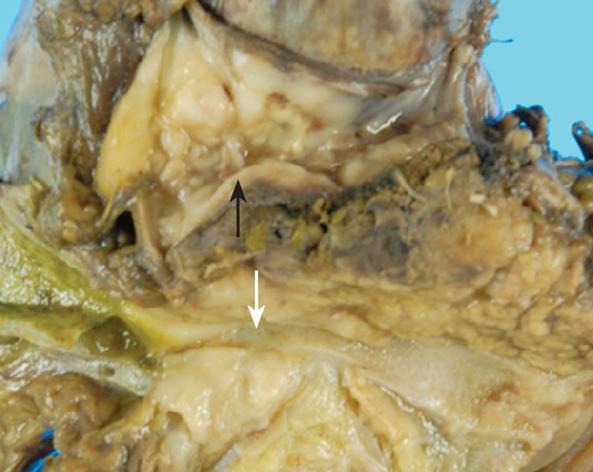
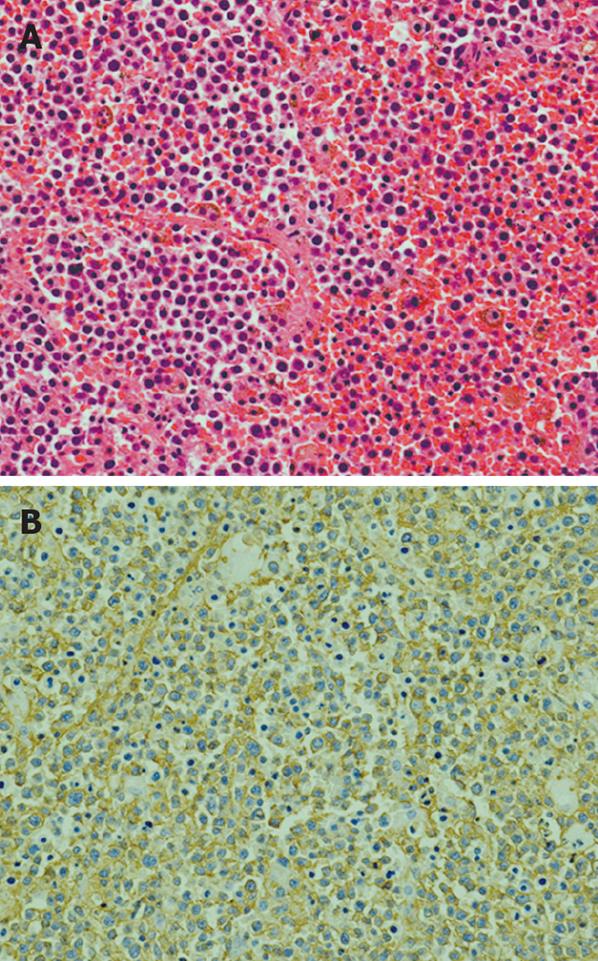
On October 22, 2008, a pylorus-preserving pancreaticoduodenectomy combined with a PV resection was performed. The PV was reconstructed using an autologous right internal jugular vein graft. The resected specimen showed endoluminal invasion of both the bile duct and the PV (Figure 2). Histological examination showed the mass consisting of diffuse sheets of large malignant lymphoid cells (Figure 3A). Immunohistochemical studies revealed that these cells were positive for CD20 (B-cell associated antigen, Figure 3B) and CD79a (B-cell associated antigen), and partially positive for CD10 (B-cell associated antigen), and negative for CD3 (T-cell associated antigen), CD4 (T-cell associated antigen) CD5 (T-cell associated antigen), CD8 (T-cell associated antigen) and CD30 (a universal feature of anaplastic large cell lymphoma). The pathologic diagnosis was diffuse large B-cell type non-Hodgkin lymphoma. The patient was transferred to the Department of Hematology and Oncology.
On January 9, 2008, combined chemotherapy including cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab (CHOP-R) was started. During the next 4 mo, he received 4 cycles of CHOP-R, and 3 cycles of combination of intrathecal chemoprophylaxis including methotrexate, cytosine arabinoside, and prednisone. The patient is alive with no evidence of the disease for 7 mo after the operation, and will receive additional courses of chemotherapy.
Because of its rarity, non-Hodgkin lymphoma is seldom considered as a differential diagnosis in patients presenting with jaundice[2]. In one retrospective study by Ravindra et al, 6 of 9 patients with non-Hodgkin lymphoma presenting obstructive jaundice underwent laparotomy before the diagnosis was made as in the present case[2], whereas, the other 3 patients were diagnosed by biopsy and could avoid laparotomy due to the following clinical features. The first patient with obstructive jaundice also had cervical lymphadenopathy, and cervical lymph node biopsy was performed, the second patient was considered too old to withstand the operation, and underwent an ultrasonographically guided percutaneous biopsy, and the third patient had a history of lymphoma and jaundice 1 year after complete remission, which suggested recurrence[2]. In our case, the patient had none of such clinical findings as described above. Furthermore, our case presented with not only obstructive jaundice but also simultaneous portal venous obstruction. Such clinical features were not observed in the Ravindra's cases[2]. The common etiology of malignant biliary obstruction occurring simultaneously with extrahepatic PV invasion is generally pancreatic carcinoma or cholangiocarcinoma[5], and we had no idea of lymphoma for a differential diagnosis in this case before operation.
Chemotherapy is the mainstay of treatment for non-Hodgkin lymphoma[67]. Surgical intervention delayed the initiation of chemotherapy in the present case, which could miss an opportunity to cure the disease. Although it remains unclear whether chemotherapy should precede the biliary drainage procedures in patients with non-Hodgkin lymphoma presenting with jaundice, chemotherapy alone usually alleviate the obstructive jaundice without biliary drainage[7]. Dudgeon et al described five patients with non-Hodgkin lymphoma causing obstructive jaundice, who were treated with combined chemotherapy without prior surgical or endoscopic biliary decompression, or radiation therapy. Jaundice was relieved rapidly (within 2-59 d) in all the patients with acceptable toxic effects, and all the five patients achieved remissions[8].
Fine needle aspiration (FNA) with percutaneous or endoscopic ultrasound (EUS) guidance should precede the operation in the present case. FNA with percutaneous or EUS guidance permits both morphologic and cytologic analysis of lesions at various locations such as within or adjacent to the gastrointestinal tract, and intra-abdominal and retroperitoneal masses[9–11]. Erickson and colleagues studied 18 patients with retroperitoneal lesions who underwent EUS and EUS-guided FNA[9]. EUS-guided FNA was done in 15 (83%) of the 18 patients, among whom four were diagnosed as having lymphomas and avoided surgical intervention[9], whereas, one patient ultimately had to have exploratory surgery for biopsy because neither EUS-guided FNA nor CT-guided percutaneous biopsy could provide enough tissues for definitive classification of a follicular centroblastic centrocytic lymphoma[9]. Similarly, in one retrospective study, 9 patients with non-Hodgkin lymphoma presented with obstructive jaundice[2]. Among them, two underwent percutaneous biopsy, and both avoided laparotomy.
One disadvantage of FNA with percutaneous or EUS guidance is a risk of complications. The possible complications associated with FNA included infection, intracystic hemorrhage, retroperitoneal hematoma, and pancreatitis[1011]. Eloubeidi et al studied 547 patients who underwent EUS-guided FNA in the preoperative evaluation of suspected pancreatic cancer[10]. In their report, 11 (2%) patients developed a major complication, and one patient required surgical debridement for necrosis[10]. Guo et al reported that one (1.5%) of 68 patients had a minor complication (hematoma) from the radiologically guided percutaneous FNA biopsy of pelvic and retroperitoneal masses[11].
Another disadvantage of FNA with percutaneous or EUS guidance is a risk of tumor seeding along a needle tract[1213]. Although the EUS-guided FNA is considered to have a lower risk of peritoneal carcinomatosis compared with percutaneous one[12], a case of tumor seeding of a pancreatic adenocarcinoma because of EUS-guided FNA has been reported[13]. Therefore, FNA with percutaneous or EUS guidance should be indicated only when additional information accessible by the procedure significantly affects the subsequent management of patients.
| 1. | Odemis B, Parlak E, Basar O, Yuksel O, Sahin B. Biliary tract obstruction secondary to malignant lymphoma: experience at a referral center. Dig Dis Sci. 2007;52:2323-2332. [Cited in This Article: ] |
| 2. | Ravindra KV, Stringer MD, Prasad KR, Kinsey SE, Lodge JP. Non-Hodgkin lymphoma presenting with obstructive jaundice. Br J Surg. 2003;90:845-849. [Cited in This Article: ] |
| 3. | Yoneyama F, Nimura Y, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Oda K. Primary lymphoma of the liver with bile duct invasion and tumoral occlusion of the portal vein: report of a case. J Hepatol. 1998;29:485-488. [Cited in This Article: ] |
| 4. | Unlu Y, Tekin SB, Ceviz M, Balci A. A successful right axillary artery graft to repair a ruptured axillary artery due to the involvement of lymphoma: report of a case. Surg Today. 2003;33:72-74. [Cited in This Article: ] |
| 5. | Gulliver DJ, Baker ME, Cheng CA, Meyers WC, Pappas TN. Malignant biliary obstruction: efficacy of thin-section dynamic CT in determining resectability. AJR Am J Roentgenol. 1992;159:503-507. [Cited in This Article: ] |
| 6. | Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, Glick JH, Coltman CA Jr, Miller TP. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-1006. [Cited in This Article: ] |
| 7. | Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242. [Cited in This Article: ] |
| 8. | Dudgeon DJ, Brower M. Primary chemotherapy for obstructive jaundice caused by intermediate-grade non-Hodgkin lymphoma. Cancer. 1993;71:2813-2816. [Cited in This Article: ] |
| 9. | Erickson RA, Tretjak Z. Clinical utility of endoscopic ultrasound and endscopic ultrasound-guided fine needle aspiration in retroperitoneal neoplasms. Am J Gastroenterol. 2000;95:1188-1194. [Cited in This Article: ] |
| 10. | Eloubeidi MA, Varadarajulu S, Desai S, Shirley R, Heslin MJ, Mehra M, Arnoletti JP, Eltoum I, Wilcox CM, Vickers SM. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg. 2007;11:813-819. [Cited in This Article: ] |
| 11. | Guo Z, Kurtycz DF, De Las Casas LE, Hoerl HD. Radiologically guided percutaneous fine-needle aspiration biopsy of pelvic and retroperitoneal masses: a retrospective study of 68 cases. Diagn Cytopathol. 2001;25:43-49. [Cited in This Article: ] |
| 12. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [Cited in This Article: ] |
| 13. | Paquin SC, Gariepy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [Cited in This Article: ] |