Published online Apr 14, 2008. doi: 10.3748/wjg.14.2235
Revised: January 30, 2008
Published online: April 14, 2008
AIM: To explore and compare the radiochemical behavior and biological property of anti-sense oligonucleotide (ASON) labeled with technetium-99m using N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycline (NHS-MAG3) and hydrazinonictinamide derivative (HYNIC).
METHODS: After HYNIC and NHS-MAG3 were synthesized, ASON was labeled with technetium-99m using HYNIC and NHS-MAG3 as a bifunctional chelator. The in vivo and in vitro stability, binding rates of labeled compounds to serum albumen, biodistribution of 99mTc-MAG3-ASON and 99mTc-HYNIC-ASON in BALB/C mouse and its HT29 tumor cellular uptake were compared.
RESULTS: The labeling efficiency and stability of 99mTc-MAG3-ASON were significantly higher than those of 99mTc-HYNIC-ASON (P = 0.02, and P = 0.03, respectively). 99mTc-MAG3-ASON had a significantly lower rate of binding to serum albumen than 99mTc-HYNIC-ASON (P < 0.05). In contrast to 99mTc-HYNIC-ASON, the biodistribution of 99mTc-MAG3-ASON was significantly lower in blood, heart, liver and stomach (P < 0.05), slightly lower in intestines and spleen (P > 0.05) and significantly higher in lung and kidney (P < 0.05). The HT29 tumor cellular uptake rate of 99mTc-MAG3-ASON was significantly higher than that of 99mTc-HYNIC-ASON (P < 0.05).
CONCLUSION: 99mTc-MAG3-ASON shows superior radiochemical behaviors and biological properties than 99mTc-HYNIC-ASON. 99mTc-MAG3-ASON is a potential radiopharmaceutical agent for in vivo application.
- Citation: Li YC, Tan TZ, Zheng JG, Zhang C. Anti-sense oligonucleotide labeled with technetium-99m using hydrazinonictinamide derivative and N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycline: A comparison of radiochemical behaviors and biological properties. World J Gastroenterol 2008; 14(14): 2235-2240
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2235.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2235
Different drugs can be used in anti-sense therapy, among which synthetic anti-sense oligonucleotide (ASON) is used to bind to deoxyribonucleic acid (DNA) translation or transcription in a sequence-specific manner and interfere with the expression of oncogene. However, it is still difficult for ASON to target tumor cells and transport across cell membrane. Besides, because of multi-gene expressions in tumor cells, inhibition of any single target gene is not sufficient to inhibit tumor growth. Radio-labeled ASON targeting specific oncogenes can overcome these problems by direct inhibition of anti-sense and radiation damage. The curative effect of radionuclide anti-sense therapy is closely related to the labeling efficacy of ASON and the characteristics of labeled compounds. In contrast to 188Re, 186Re, 90Y[1–3], hydrazinonictinamide derivative (HYNIC) and N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycline (NHS-MAG3), as a bifunctional chelator, have been known to help label ASON[4–6] with 99mTc. However, few reports are available on the comparison of both chelators. This study was to compare the radiochemical behaviors and biological properties of ASON labeled with technetium-99m using NHS-MAG3 and HYNIC.
BALB/c nude mice at the age of 6-8 wk, weighing 17-22 g, were obtained from West China Experimental Animal Center. Human colon carcinoma HT29 cell line was obtained from the Laboratory of West China Hospital. HT29 cells were incubated in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 &mgr;g/mL streptomycin. Fifteen-mer phosphorothioate ASON (5’-NH2-FACGTTGAGGGGCAT-3’, F is adenosine sulfurised), which is complementary to the translation start site of c-myc mrRNA, was purchased from GiBcoBRL (USA). 99mTcO4- (37 TBq/L) was obtained from Chengdu Gaotong Isotope Corporation (Chengdu, China). Sephadex G25 was from Pharmacia Fine Chemicals A.B (Uppsala, Sweden). C18 Sep-Pak reversed-phase column was a product from Waters Company (Milford, USA). CRC-15R dose calibrator was from Capintec Company (Ramsey, New Jersey, USA). FH463A automatic scaler was supplied by Beijing Nuclear Instrument Company (Beijing, China). Unity Inova-400 nuclear magnetic resonator was from Varian Company (USA). UV-2100 spectrophotometer was from Beckman Company (Cotati, California, USA). Frozen desiccator was from Marathon Electric Company (New York, USA). CO2 incubator was from Sanyo Company (Japan). Centrifuge was from Beckman Company (Cotati, California, USA).
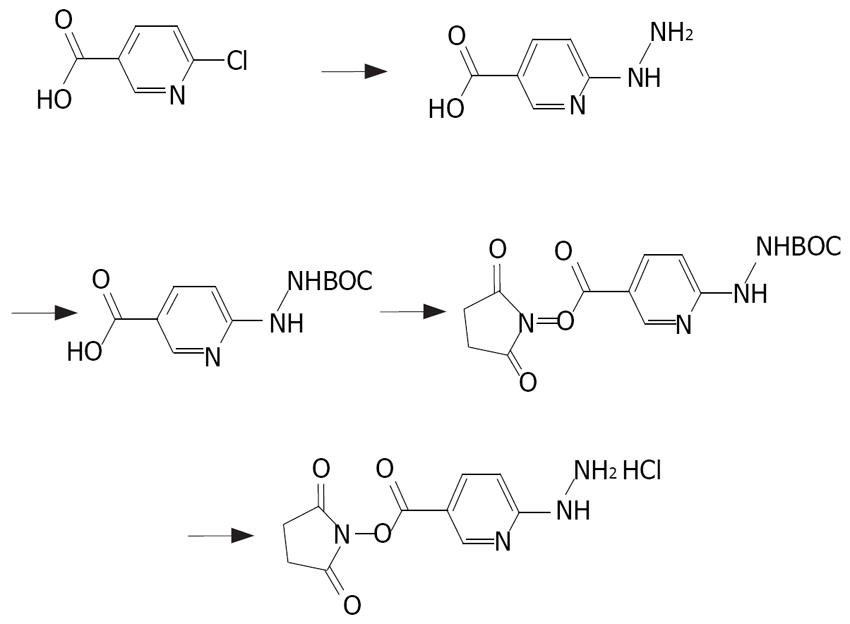
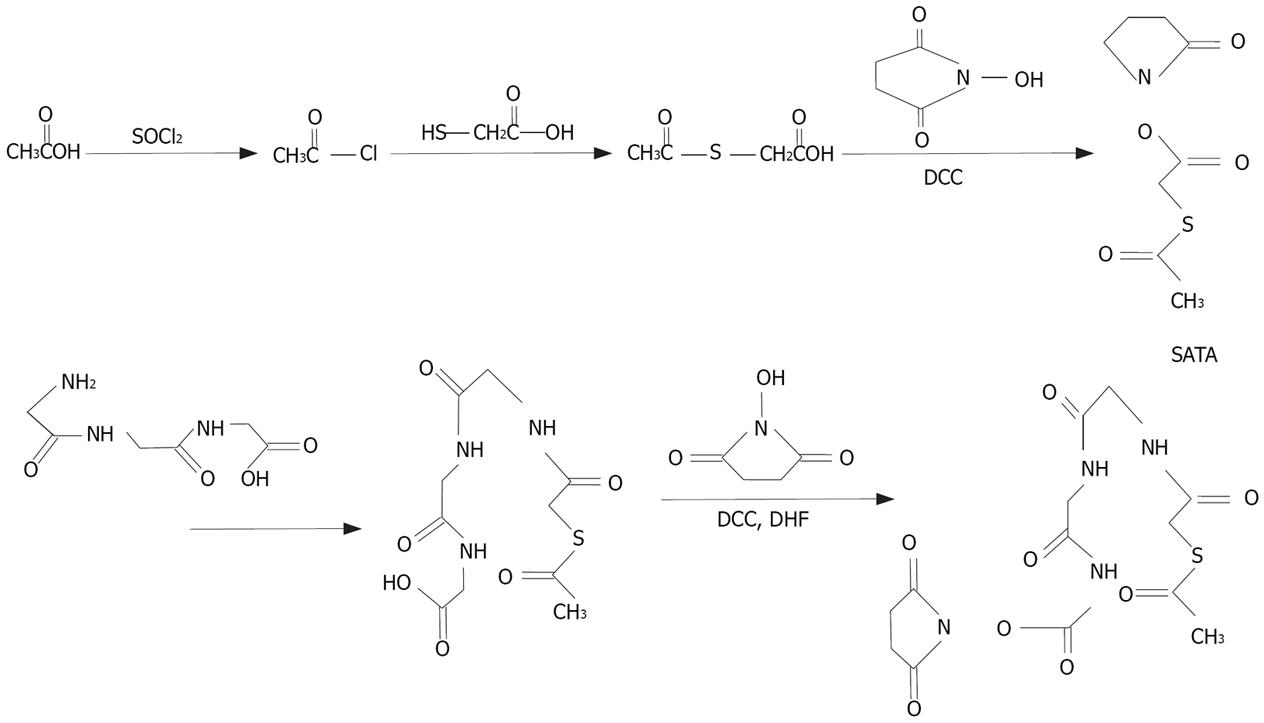
HYNIC was synthesized as previously described[7] (Figure 1). The end product was purified by recrystalli-zation in isopropyl alcohol and the yield was 75%. The synthesis process of NHS-MAG3 has been described elsewhere[6] (Figure 2). The melting temperature of the end product was 140°C-155°C, the yield was 80%. The content was 2.38 ppm (S, 3H, SCOCH3), 2.80 ppm (S, 4H, succinimidyl), 3.68-3.80 ppm (M, 8H, COCH2) and 8.20-8.38 ppm (M, 3H, NHCO), respectively, by nuclear magnetic resonance spectroscopy.
ASON (2 mg/mL) buffer was dissolved in 2 mol/L NaCl, 0.5 mol/L NaHCO3 and 2 mmol/L ethylenediamine tetraacetic acid (EDTA), and HYNIC (10 mg/mL) was dissolved in dimethylformamide (DMF). In 45°C water bath, 31 &mgr;L HYNIC and 2 mmol/L EDTA were gradually dropped into a 25 &mgr;L ASON solution at the molar ratio of 25:1. The reaction system was filtered through a Sep-Pak C18 reversed-phase column (10 mm × 5 mm) in 60% methanol to remove HYNIC not binding to ASON. HYNIC-ASON was collected with a UV-2100 spectrophotometer and frosted to dry powder for storage. HYNIC-ASON dry powder was labeled on d 15, 30 or 60, respectively. Ten &mgr;g HYNIC-ASON powder was dissolved in a 0.5 mL tricine solution (70 mg/mL). SnCl2·2H2O solution (1 mg/mL) was dissolved in 0.1 mol/L HCl at room temperature. HYNIC-ASON solution, 25 &mgr;L SnCl2·2H2O solution and 0.2 mL 99mTcO4- containing a radioactivity of 370, 740 or 1480 MBq were mixed uniformly. After stored for 30 min at room temperature, the mixture was eluted and purified through a Sep-Pak C18 reversed-phase column (10 mm × 5 mm) in 60% methanol, and 99mTc-HYNIC-ASON solution was collected. Chromatographic assay was performed in both solution systems before and after purification to detect the labeling efficacy and radio-chemical purity of 99mTc-HYNIC-ASON, where Xihua I filter paper as a sustentaculum was developed with 85% methanol as a developer.
Twenty-five microliter ASON (2 mg/mL, dissolved in 0.25 mol/L NaHCO3 and 1 mol/L EDTA, pH = 8.5) was mixed with 42 &mgr;L NHS-MAG3 (10 mg/mL, dissolved in dimethylsulphoxide) at the molar ratio of 1:25. The mixture reacted at room temperature in the dark for 15 min. Any free NHS-MAG3 was removed through Sep-Pak C18 reversed-phase column (10 mm × 5 mm) in 60% methanol. The bound MAG3-ASON was collected with a spectrophotometer and frosted to dry powder for storage. The target-bound complex, dry powder on d 15, 30 or 60 at room temperature, was labeled. Fifty &mgr;L MAG3-ASON (1 mg/mL) in re-distilled water was mixed with 10 &mgr;L NaHCO3 (0.5 mol/L)-sodium tartrate (50 mg/mL) buffer (pH = 9.2). Ten &mgr;L SnCl2·2H2O fresh solution (1 mg/mL, dissolved in 0.1 mol/L HCl) was dropped into MAG3-ASON at room temperature and mixed uniformly. At last, 0.2 mL 99mTcO4- (containing a radioactivity of 370, 740 or 1480 MBq) was added into the above solutions, respectively. After 15 min, 99mTc-ASON-MAG3 was purified on Sephadex G25 column (250 mm × 5 mm) in an ammonium acetate solution (0.25 mol/L, pH = 5.2) and collected. Chromatographic assay was performed in both specimens before and after purification to evaluate the labeling efficiency and radiochemical purity. A system was demanded to develop Xihua I filter paper in 85% methanol.
The stability of labeled compounds was assessed for 1, 2 and 4 h, respectively, at room temperature, by measuring the radiochemical purity on Xihua I filter paper that was developed in 85% methanol.
Three hundred and seventy mL MBq 99mTc-HYNIC-ASON or 99mTc-MAG3-ASON was mixed with 2 mL anti-coagulated rabbit fresh plasma for 6 cuvettes. After incubated at 37 °C for 2 h, the mixture was mixed with 5 mL trichloroacetic acid (250 g/L) and centrifuged for 5 min at 1200 ×g. The precipitate was washed twice with 2 mL trichloroacetic acid (250 g/L) and the supernatant was collected. The radioactivity of precipitate and supernatant was measured, respectively. The binding rate of 99mTc-HYNIC-ASON or 99mTc-MAG3-ASON to rabbit plasma protein was calculated by the following formula: Binding rate (%) = radioactivity of precipitate/radioactivity of precipitate and supernatant.
99mTc-HYNIC-ASON or 99mTc-MAG3-ASON (0.2 mL, 148 KBq) was separately injected into the tail veins of 20 BALB/c nude mice (age: 6-8 wk, body weight: 17-22 g) which were randomly divided into four groups (5 in each group). Mice in each group were sacrificed at 0.5, 1, 2 and 4 h, respectively, after injection of 99mTc-HYNIC-ASON or 99mTc-MAG3-ASON. Blood, heart, lungs, liver, kidneys, spleen, stomach, intestine and muscles were removed and weighed. The tissue uptake rate of labeled compounds was calculated according to the following equation: tissue uptake rate (%ID/g) = radioactivity of per gram of wet tissue weight/radioactivity of wet tissue injected into the body. The results were expressed as percentage of radioactivity within per gram of wet tissue.
Human colon carcinoma HT29 cells were incubated with RPMI 1640 medium containing 10% fetal calf serum, 100 U/mL penicillin and 100 &mgr;g/mL streptomycin. Tumor cells were cultured in 80 wells of 96-well plates (1.5 × 106 cells/well). It took about 24 h for cells to adhere to wells. The culture medium was pipetted and 2 mL serum-free RPMI 1640 medium containing 74 KBq 99mTc-HYNIC-ASON or 99mTc-MAG3-ASON was added to each of the 80 wells. The cells were incubated in a humidified incubator containing 50 mL/L CO2 at 37°C for 10, 20, 40, 60 and 120 min, respectively. Each well was rinsed 3 times with RPMI 1640 medium. At last, all the human colon carcinoma HT29 cells and supernatant in each well were collected and the radioactivity was calculated. The following formula was used to calculate the percentage of radioactivity within the cells of each well: cellular uptake rate (%) = radioactivity absorbed in each well/radioactivity added to each well.
The data were expressed as mean ± SD and input into a computer for statistical analysis with SPSS 11 software. Differences among the groups were compared with paired t-test. P < 0.05 was considered statistically significant.
Analysis of labeling efficiency and radiochemical purity of the labeled compounds showed that the flow rate of 99mTc-HYNIC-ASON and 99mTc-MAG3-ASON, 99mTcO4-, and deoxidized technetium, was 0.9-1.0, 0.6-0.7, and 0-0.1, respectively. The labeling efficiency and radiochemical purity of labeled compounds are listed in Table 1. The labeling efficiency of 99mTc via NHS-MAG3 was higher than that of 99mTc via HYNIC (for interval between binding and labeling: t = 6.715, P = 0.021; for radioactivity of 99mTcO4-: t = 11.736, P = 0.007). The radioactivity of 99mTcO4- hardly influenced the labeling efficiency. The radiochemical purity of labeled compounds was higher than 95% and there was no statistical difference between the two methods (for interval between binding and labeling: t = -0.444, P = 0.701; for radioactivity of 99mTcO4-: t = 2.656, P = 0.117). Either interval between binding and labeling of HYNIC-ASON or that of MAG3-ASON had almost no effect on the labeling efficiency and radiochemical purity of labeled compounds.
| Interval between binding and labeling (d) | Radioactivity of 99mTcO4- (MBq) | |||||
| 15 | 30 | 60 | 370 | 740 | 1480 | |
| Labeling efficiency (%) | ||||||
| Via HYNIC | 57.36 ± 3.69 | 62.13 ± 4.25 | 62.87 ± 3.04 | 58.74 ± 5.32 | 62.86 ± 4.27 | 63.28 ± 3.38 |
| Via NHS-MAG3 | 67.35 ± 4.03 | 68.35 ± 3.56 | 69.85 ± 4.63 | 68.67 ± 4.82 | 70.31 ± 5.09 | 71.56 ± 5.37 |
| Radiochemical purity (%) | ||||||
| 99mTc-HYNIC-ASON | 95.75 ± 5.21 | 96.32 ± 4.92 | 95.86 ± 5.28 | 96.56 ± 4.45 | 96.87 ± 3.65 | 97.16 ± 4.34 |
| 99mTc-MAG3-ASON | 96.43 ± 4.69 | 95.67 ± 5.17 | 96.39 ± 4.78 | 96.35 ± 6.12 | 95.86 ± 4.67 | 96.54 ± 5.65 |
To assess the radiochemical purity, the labeled compounds were incubated at room temperature or at 37°C after diluted with an equal volume of fresh human serum (Table 2). The radiochemical purity of 99mTc-MAG3-ASON was much higher than that of 99mTc-HYNIC-ASON (at room temperature: t = 5.616, P = 0.030; at 37°C: t = 5.616, P = 0.032), while the radiochemical purity of 99mTc-MAG3-ASON was less affected by incubation time than that of 99mTc-HYNIC-ASON.
| Incubation time at room temperature (h) | Incubation time at 37°C (h) | |||||
| 1 | 2 | 4 | 1 | 2 | 4 | |
| 99mTc-HYNIC-ASON | 93.43 ± 5.32 | 89.17 ± 4.62 | 87.16 ± 5.36 | 92.75 ± 4.46 | 89.52 ± 3.67 | 86.86 ± 5.49 |
| 99mTc-MAG3-ASON | 97.26 ± 6.02 | 96.68 ± 5.54 | 96.39 ± 4.68 | 95.86 ± 5.69 | 95.47 ± 4.07 | 94.79 ± 5.34 |
The binding rate of rabbit serum protein for 99mTc-MAG3-ASON or 99mTc-HYNIC-ASON was 11.17% ± 1.31% and 71.06% ± 3.56%, respectively. The differences between them were statistically significant, and the former was lower than the latter (t = 27.346, P < 0.0001).
The biodistributions of 99mTc-MAG3-ASON and 99mTc-HYNIC-ASON in BALB/c mice are listed in Table 3. The distributions of 99mTc-MAG3-ASON were significantly lower in blood, heart, liver and stomach than those of 99mTc-HYNIC-ASON (P < 0.05). The distributions of 99mTc-MAG3-ASON were significantly higher in lungs and kidneys than those of 99mTc-HYNIC-ASON (P < 0.05). There was no statistical difference in the distributions of 99mTc-MAG3-ASON and 99mTc-HYNIC-ASON in spleen, intestines and muscle.
| Tissue | 0.5 h | 1 h | 2 h | 4 h | Paired | t-test | ||||
| M | H | M | H | M | H | M | H | |||
| Blood | 1.12 ± 0.76 | 6.21 ± 1.03 | 2.38 ± 0.63 | 6.56 ± 1.11 | 1.14 ± 0.42 | 3.58 ± 1.21 | 1.10 ± 0.09 | 2.83 ± 0.54 | t = 4.347 | P = 0.022 |
| Heart | 0.62 ± 0.31 | 2.13 ± 0.45 | 0.58 ± 0.07 | 1.64 ± 0.34 | 0.32 ± 0.05 | 1.16 ± 0.12 | 0.18 ± 0.08 | 0.81 ± 0.13 | t = 5.362 | P = 0.013 |
| Lungs | 3.11 ± 0.82 | 2.68 ± 0.65 | 3.87 ± 1.36 | 3.23 ± 1.04 | 3.04 ± 0.79 | 2.78 ± 1.03 | 2.08 ± 0.62 | 1.76 ± 0.36 | t = -4.934 | P = 0.016 |
| Liver | 7.52 ± 2.45 | 11.46 ± 2.31 | 13.19 ± 1.47 | 15.24 ± 2.53 | 9.21 ± 1.03 | 12.89 ± 1.68 | 9.48 ± 2.56 | 10.46 ± 1.97 | t = 3.806 | P = 0.032 |
| Kidneys | 11.42 ± 3.34 | 4.17 ± 1.05 | 17.13 ± 2.86 | 5.03 ± 0.94 | 24.58 ± 3.57 | 2.78 ± 0.68 | 21.95 ± 4.02 | 2.28 ± 0.95 | t = -4.511 | P = 0.020 |
| Spleen | 2.71 ± 1.62 | 4.87 ± 2.36 | 5.65 ± 0.93 | 6.08 ± 1.93 | 5.35 ± 0.26 | 4.08 ± 1.54 | 4.84 ± 1.33 | 5.21 ± 2.04 | t = 0.603 | P = 0.589 |
| Stomach | 1.08 ± 0.86 | 7.46 ± 2.13 | 2.58 ± 0.95 | 11.48 ± 3.01 | 1.73 ± 0.21 | 7.49 ± 1.86 | 1.41 ± 0.34 | 7.85 ± 3.02 | t = 9.901 | P = 0.002 |
| Intestines | 0.53 ± 0.31 | 1.26 ± 0.31 | 0.86 ± 0.14 | 2.68 ± 0.95 | 1.23 ± 0.19 | 6.44 ± 2.13 | 1.96 ± 0.53 | 7.84 ± 2.34 | t = 2.706 | P = 0.073 |
| Muscle | 1.35 ± 0.16 | 0.54 ± 0.21 | 0.87 ± 0.63 | 0.94 ± 0.81 | 0.75 ± 0.08 | 0.42 ± 0.06 | 0.64 ± 0.15 | 0.19 ± 0.05 | t = -2.095 | P = 0.127 |
Cellular uptake of labeled compounds in human colon carcinoma HT29 cells is listed in Table 4. The cellular uptake of 99mTc-MAG3-ASON was significantly higher than that of 99mTc-HYNIC-ASON (t = 3.770, P = 0.020), which was 6.5, 10.1, 8.4, 9.5 and 9.1-folds higher than those of 99mTc-HYNIC-ASON at 10, 20, 40, 60 and 120 min after incubation.
| 10 min | 20 min | 40 min | 60 min | 120 min | |
| 99mTc-HYNIC-ASON (%) | 0.43 ± 0.08 | 0.56 ± 0.21 | 0.93 ± 0.54 | 1.42 ± 0.64 | 1.67 ± 0.86 |
| 99mTc-MAG3-ASON (%) | 2.78 ± 0.81 | 5.64 ± 0.51 | 7.82 ± 2.53 | 13.63 ± 2.71 | 15.25 ± 3.13 |
In our studies, because expensive acetylsulfoacetic acid was not available, S-acetylthioglycolic acid N-hydroxysuccinimide ester (SATA) was synthesized as previously described[5]. The synergenic coligand of tricine (N-tris-hydroxy-methyl-methylglycine) was applied to the synthesis of HYNIC, to achieve the high radioactivity of labeled compounds. During the synthesis of labeled compounds, isopropylol was used to crystallize the compounds instead of chromatographic column purification. Their synthesis was simple, efficient, economical, with a high yield (75%-80%) and little environmental pollution. Nuclear magnetic resonance of labeled compounds was performed as previously described[5–7]. Both Sep-Pak C18 reversed-phase column and Sephadex G25 column could be used to purify the radiolabeled ASON. Both labeling methods can achieve a high radiochemical purity of over 95%.
Stability can be obtained by methylation, amination or sulfonation of the phosphorus atoms in ASON, making it not recognized and degradated by nucleic acid enzyme[8]. In the present study, we modified the ASON by replacing the hydroxyl group in the phosphoric acid branch of ASON with a sulphur atom and attaching an amid to the 5′ terminal of ASON. Labeled compounds were observed for four hours to detect the stability of ASON labeled with 99mTc via HYNIC or NHS-MAG3. Only 1-2 covalent bonds were formed between a molecule of HYNIC-ASON and a technetium atom. However, it was reported that 4-5 covalent bonds can form between MAG3-ASON and technetium[910], which may be the reason for a greater stability of 99mTc-MAG3-ASON than that of 99mTc-HYNIC-ASON. During labeling, since the mercapto group of ASON-MAG3 is protected by acetyl group, excessive SnCl2 is needed to hydrolyze the protection group of ASON-MAG3[11], which may be the reason for a greater labeling efficiency of 99mTc via NHS-MAG3 than via HYNIC.
The binding rate of 99mTc-MAG3-ASON to rabbit serum protein was significantly lower than that of 99mTc-HYNIC-ASON in our study, suggesting that the distributions of 99mTc-MAG3-ASON are significantly lower in blood, heart and liver of BALB/c mice. The distributions of 99mTc-MAG3-ASON were much lower in stomach than those of 99mTc-HYNIC-ASON, suggesting that 99mTc-MAG3-ASON has a greater in vivo ability than 99mTc-HYNIC-ASON. The distributions of 99mTc-MAG3-ASON were much higher in kidneys than those of 99mTc-HYNIC-ASON, which may be related to the metabolism of MAG3-ASON in kidneys.
Cellular targeting uptake of ASON can be improved by receptor-mediated mechanisms[12–14]. The conjugation of vasoactive intestinal peptide (VIP)-ASON is very helpful for 125I-ASON to selectively bind to HT29 tumor cells by VIP receptors. For such tumor cells that highly express VIP receptors, tumor cellular uptake of VIP-125I-ASON is significantly higher than that of 125I-ASON un-conjugated to VIP[12]. The c-myc ASON complex entered human melanoma cells (M14) by folacin receptors on tumor cell surface, brings about a greater cellular uptake than that of free-ASON, and inhibits tumor growth by lowing c-myc cancer protein expression[13]. As we know, the c-myc oncogene and transferrin receptors are highly expressed in HL-60 and LoVo Dx cells, the addition of transferrin-polylysine-c-myb ASON complex would cause more tumor cell deaths than free c-myb ASON[14]. However, receptor mediation was not used in our study. Why HT29 cellular uptake of 99mTc-MAG3-ASON is higher than that of 99mTc-HYNIC-ASON is unclear, which is possibly related to the greater stability of 99mTc-MAG3-ASON, and needs further study.
Anti-sense oligonucleotide (ASON) is used to bind to deoxyribonucleic acid (DNA) translation or transcription and interfere with the expression of oncogene. However, ASON is not sufficient to inhibit tumor growth. In order to enhance anti-tumor effect of ASON, we labeled ASON with technetium-99m via N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycline (NHS-MAG3) and hydrazinonictinamide derivative (HYNIC).
Many proteins such as monoclonal antibody, polypeptide, ligand, can be labeled with radionuclides, such as 125I, 131I, 32P, 35S, 99mTc, 188Re, 186Re, 90Y. We are trying to label oncolytic virus with radionuclide, in order to achieve a synergistic anticancer effect.
In this study, we compared the radiochemical behaviors and biological properties of anti-sense oligonucleotide (ASON) labeled with technetium-99m via N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycline (NHS-MAG3) and hydrazinonictinamide derivative (HYNIC) and found that 99mTc-MAG3-ASON showed superior radiochemical behaviors and biological properties than 99mTc-HYNIC-ASON.
99mTc-MAG3-ASON showed superior radiochemical behaviors and biological properties than 99mTc-HYNIC-ASON, suggesting that it can be used as a potential radiopharmaceutic agent for in vivo application.
In this study, the authors analyzed and compared the radiochemical behaviors and biological properties of anti-sense oligonucleotide (ASON) labeled with technetium-99m via NHS-MAG3 and HYNIC. The rationale of the study is clearly expressed and the experiments appear to be carefully conducted.
| 1. | Wessels BW, Rogus RD. Radionuclide selection and model absorbed dose calculations for radiolabeled tumor associated antibodies. Med Phys. 1984;11:638-645. [Cited in This Article: ] |
| 2. | Hnatowich DJ, Virzi F, Doherty PW. DTPA-coupled antibodies labeled with yttrium-90. J Nucl Med. 1985;26:503-509. [Cited in This Article: ] |
| 3. | Anderson-Berg WT, Squire RA, Strand M. Specific radio-immunotherapy using 90Y-labeled monoclonal antibody in erythroleukemic mice. Cancer Res. 1987;47:1905-1912. [Cited in This Article: ] |
| 4. | Hnatowich DJ, Mardirossian G, Fogarasi M, Sano T, Smith CL, Cantor CR, Rusckowski M, Winnard P Jr. Comparative properties of a technetium-99m-labeled single-stranded natural DNA and a phosphorothioate derivative in vitro and in mice. J Pharmacol Exp Ther. 1996;276:326-334. [Cited in This Article: ] |
| 5. | Winnard P Jr, Chang F, Rusckowski M, Mardirossian G, Hnatowich DJ. Preparation and use of NHS-MAG3 for technetium-99m labeling of DNA. Nucl Med Biol. 1997;24:425-432. [Cited in This Article: ] |
| 6. | Gano L, Patricio L, Marques E, Cantinho G, Pena H, Martins T, Hnatowich DJ. Human polyclonal immunoglobulin labelled with technetium-99m via NHS-MAG3: a comparison of radiochemical behavior and biological efficacy with other labelling methods. Nucl Med Biol. 1998;25:395-403. [Cited in This Article: ] |
| 7. | Abrams MJ, Juweid M, tenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss HW, Fischman AJ. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med. 1990;31:2022-2028. [Cited in This Article: ] |
| 8. | Wagner RW. Gene inhibition using antisense oligodeoxynu-cleotides. Nature. 1994;372:333-335. [Cited in This Article: ] |
| 9. | Juliano RL, Akhtar S. Liposomes as a drug delivery system for antisense oligonucleotides. Antisense Res Dev. 1992;2:165-176. [Cited in This Article: ] |
| 10. | Hnatowich DJ, Qu T, Chang F, Ley AC, Ladner RC, Rusckowski M. Labeling peptides with technetium-99m using a bifunctional chelator of a N-hydroxysuccinimide ester of mercaptoacetyltriglycine. J Nucl Med. 1998;39:56-64. [Cited in This Article: ] |
| 11. | Bryson N, Lister-James J, Jones AG, Davis WM, Davision A. Protecting groups in the preparation of thiolate complexes of technetium. Inorg Chem. 1990;29:2948-2951. [Cited in This Article: ] |
| 12. | Ou X, Tan T, He L, Li Y, Li J, Kuang A. Antitumor effects of radioiodinated antisense oligonuclide mediated by VIP receptor. Cancer Gene Ther. 2005;12:313-320. [Cited in This Article: ] |
| 13. | Ginobbi P, Geiser TA, Ombres D, Citro G. Folic acid-polylysine carrier improves efficacy of c-myc antisense oligo-deoxynucleotides on human melanoma (M14) cells. Anticancer Res. 1997;17:29-35. [Cited in This Article: ] |
| 14. | Citro G, Perrotti D, Cucco C, D'Agnano I, Sacchi A, Zupi G, Calabretta B. Inhibition of leukemia cell proliferation by receptor-mediated uptake of c-myb antisense oligodeoxynu-cleotides. Proc Natl Acad Sci USA. 1992;89:7031-7035. [Cited in This Article: ] |