Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5261
Revised: July 25, 2007
Accepted: August 19, 2007
Published online: October 21, 2007
AIM: To investigate the expression and localization of periostin in gastric cancer and its clinical relevance.
METHODS: Reverse transcriptase polymerase chain reaction was used to measure periostin mRNA expression. Western blotting was carried out to detect periostin protein expression. Immunohistochemistry was performed to localize and quantify the expression of periostin in benign gastric diseases and gastric cancer, and immunostaining results were correlated with gastric cancer pathological stages.
RESULTS: Periostin expression was low at both mRNA and protein levels in normal gastric tissues, but was overexpressed in gastric cancer tissues. Immunohistochemical staining revealed that periostin was overexpressed in primary gastric cancer, as well as in metastatic lymph nodes, but only faint staining was found in benign gastric ulcers. By quantitative analysis of the immunostaining results, periostin expression was increased 2.5-4-fold in gastric cancer, compared to that in benign gastric disease, and there was a trend toward increasing periostin expression with tumor stage.
CONCLUSION: This observation demonstrated that periostin was overexpressed in gastric cancer and lymph node metastasis, which suggests that periostin plays an important role in the progression and metastasis of gastric cancer.
- Citation: Li JS, Sun GW, Wei XY, Tang WH. Expression of periostin and its clinicopathological relevance in gastric cancer. World J Gastroenterol 2007; 13(39): 5261-5266
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5261
Gastric cancer remains the fourth most common cancer worldwide. Due to its high incidence and malignant behavior, and the lack of major advances in treatment strategy, it is still the second most frequent cause of death from malignant diseases[1]. A variety of clinicopathological characteristics may affect the prognosis of gastric cancer, and accumulating evidence has revealed that numerous genetic markers influence the biological behavior of gastric cancer. The abnormal expression of certain genes in cancer cells is closely related to various aspects of tumor progression, including tumor growth, invasion and metastasis, and inactivation of tumor suppressor genes, particularly the p53 or adenomatous polyposis coli gene[2].
Recently, great efforts have been made to determine the gene expression pattern differences between various types of human cancer and their corresponding normal tissues, although the alterations of a certain number of tumor suppressor genes and oncogenes have been shown to be closely associated with the progression of human cancer.Less is known about the functions of a large number of other genes whose expression patterns are also significantly changed during the tumorigenic process. Particularly interesting is the mesenchyme-specific gene family, normally associated with osteoblasts, which are highly expressed in various types of human cancer[3,4]. One such candidate gene is that for periostin, which is overexpresed in several types of human cancer, such as breast, colon and ovarian cancer[5-7]. Furthermore, it is supposed that, at the molecular level, periostin functions as a ligand for alpha (V), beta (3), and alpha (V) beta (5) integrins to support adhesion and migration of ovarian epithelial cells[5], and periostin activates the Akt/PKB signaling pathway through the alpha (V) beta (3) integrins, to increase cellular survival[7]. While integrin expression level is supposed to be related to the metastasis and relapse of gastric cancer[8], the expression pattern of periostin in gastric cancer is still unknown. In this paper we investigated periostin expression profile in gastric cancer and its clinical relevance.
Samples from 35 gastric cancers, including five metastatic lymph node samples, were obtained from 25 male and 10 female patients (median age, 56 years; range, 45-79 years) who underwent gastric resection at the University Hospital of the Affiliated Zhong-Da Hospital, Southeast University. Five benign gastric ulcer tissue samples were obtained from three male and two female patients, in whom gastrectomy was performed (median age, 55 years; range, 25-67 years). According to the TNM classification of the Union International Contre le Cancer (UICC), the 35 gastric cancers were classified as follows: five tumors were stageI, eight were stage II, 16 were stage III, and six were stage IV. The vast majority of the tumors were located in the distal part of the stomach (21 cases), and one-third were located in the proximal part of the stomach. Immediately upon surgical removal, tissue samples were either snap-frozen in liquid nitrogen and then maintained at 80°C until use (for RNA extraction), or fixed in 5% formalin and embedded in paraffin after 24 h. All studies were approved by the ethics committees of the Affiliated Zhong-Da Hospital, Southeast University.
Total RNA from human gastric tissues was isolated either by the Trizol Reagent (TIANGEN, Beijing, China) according to the manufacturer’s instructions. Potentially contaminating DNA was removed by RNAse-free DNAseItreatment. Primers for the genes of interest were: periostin193 bp (5'-GCCATCACATCGGACATA-3' and 5'-CTCCCATAATAGACTCAGAACA-3'), and β-actin 621 bp (5'-ACACTGTGCCCATCTACGAGG-3' and 5'-AGGGGCCGGACTCGTCATACT-3'). The RT-PCR conditions were: for the first strand synthesis of cDNA, 48°C for 45 min and 94°C for 2 min to denature the template; and for second strand synthesis and DNA amplification, 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min, for a total of 32 cycles, followed by a single step at 68°C for 7 min.
The products were visualized on 1.5% agarose gels stained with ethidium bromide, and signals were quantified by densitometry using the MetaView analyzing system (version 4.5 Universal Imaging, West Chester, PA, USA). Periostin expression was standardized to β-actin expression assessed from the same cDNA in separate PCR reactions, and run on the same gels. The standardized mean of each triplicate PCR was then expressed relative to the levels in β-actin cDNA. The density value was analyzed by Labimage software (Halle, Germany). P < 0.05 was considered as statistically significant.
Tissues were lysed in 0.5 mL lysis buffer containing 50 mmol/L Tris/HCl (pH 7.5), 150 mmol/L NaCl, 2 mmol/L EDTA (pH 8.0) and 1% SDS with proteinase inhibitors (one tablet/10 mL, Roche Molecular Biochemicals, USA). Protein concentration was determined by the BCA Protein Assay (Pierce, Rockford, IL, USA).
Fifty micrograms of protein was separated on SDS-polyacrylamide gels and electroblotted onto nitrocellulose membranes. Membranes were then incubated in blocking solution (5% non-fat milk in 20 mmol/L Tris/HCl, 150 mmol/L NaCl, 0.1% Tween-20; TBS-T), followed by incubation with rabbit anti-periostin antibodies (Abcam, Cambridge, MA, USA; 1:500) at 4°C overnight. The membranes were then washed in TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham Life Sciences, Amersham, UK) for 1 h at room temperature. Antibody detection was performed with an enhanced chemiluminescence reaction (Amersham Life Sciences).
Immunohistochemistry was performed using the strepavidin-peroxidase technique and the DAKO EnVision System (Dako Cytomation, Hamburg, Germany). Consecutive paraffin-embedded tissue sections (4-μm thick) were deparaffinized and rehydrated. Antigen retrieval was performed by pretreatment of the slides in citrate buffer (pH 6.0) in a microwave oven for 12 min. Thereafter, slides were cooled to room temperature in deionized water for 5 min. Endogenous peroxidase activity was quenched by incubating the slides in methanol containing 0.6% hydrogen peroxide, followed by washing in deionized water for 3 min, after which the sections were incubated for 1 h at room temperature with normal goat serum, and subsequently at room temperature for 1 h with the primary anti-periostin antibody (Abcam; 1:100). Next, the sections were rinsed with washing buffer (Tris-buffered saline with 0.1% BSA) and incubated with biotinylated goat anti-rabbit IgG and streptavidin-peroxidase complex, followed by reaction with diaminobenzidine and counterstaining with Mayer's hematoxylin. In addition, to confirm the specificity of the primary antibody and the technique used, tissue sections were incubated in the absence of the primary antibody and with negative control rabbit IgG. Under these conditions, no specific immunostaining was detected. The quantitative analysis of the immunohistochemistry staining of periostin expression in gastric tissues was performed as previously described[9]. The staining of each tissue was analyzed to determine the mean optical density (MOD), which represents the concentration of the stain per positive pixels.
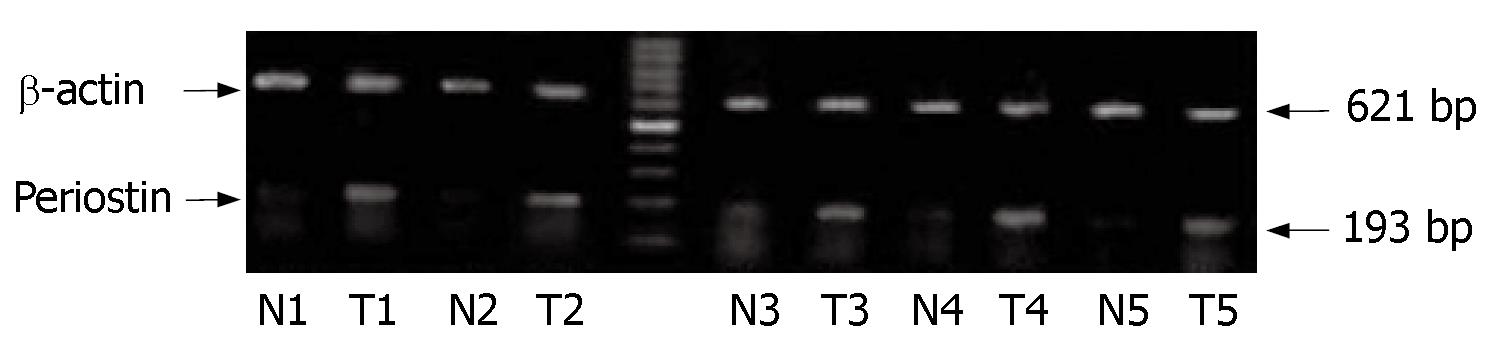
To clarify the expression of periostin in human gastric tissues, we initially examined the expression of periostin mRNA in five gastric cancer tissues (T) versus normal gastric tissues (N) obtained from the same patients. The expression of β-actin was examined as an internal control. As shown in Figure 1, periostin mRNA was expressed in all five gastric cancer tissues at different levels. In contrast, its staining was detected at a very low level in normal gastric tissues, and quantitative analysis showed that there was a significant difference in periostin expression between gastric cancer and normal tissues (P < 0.05, the density ratio between N and T was 0.1912 vs 0.8804).
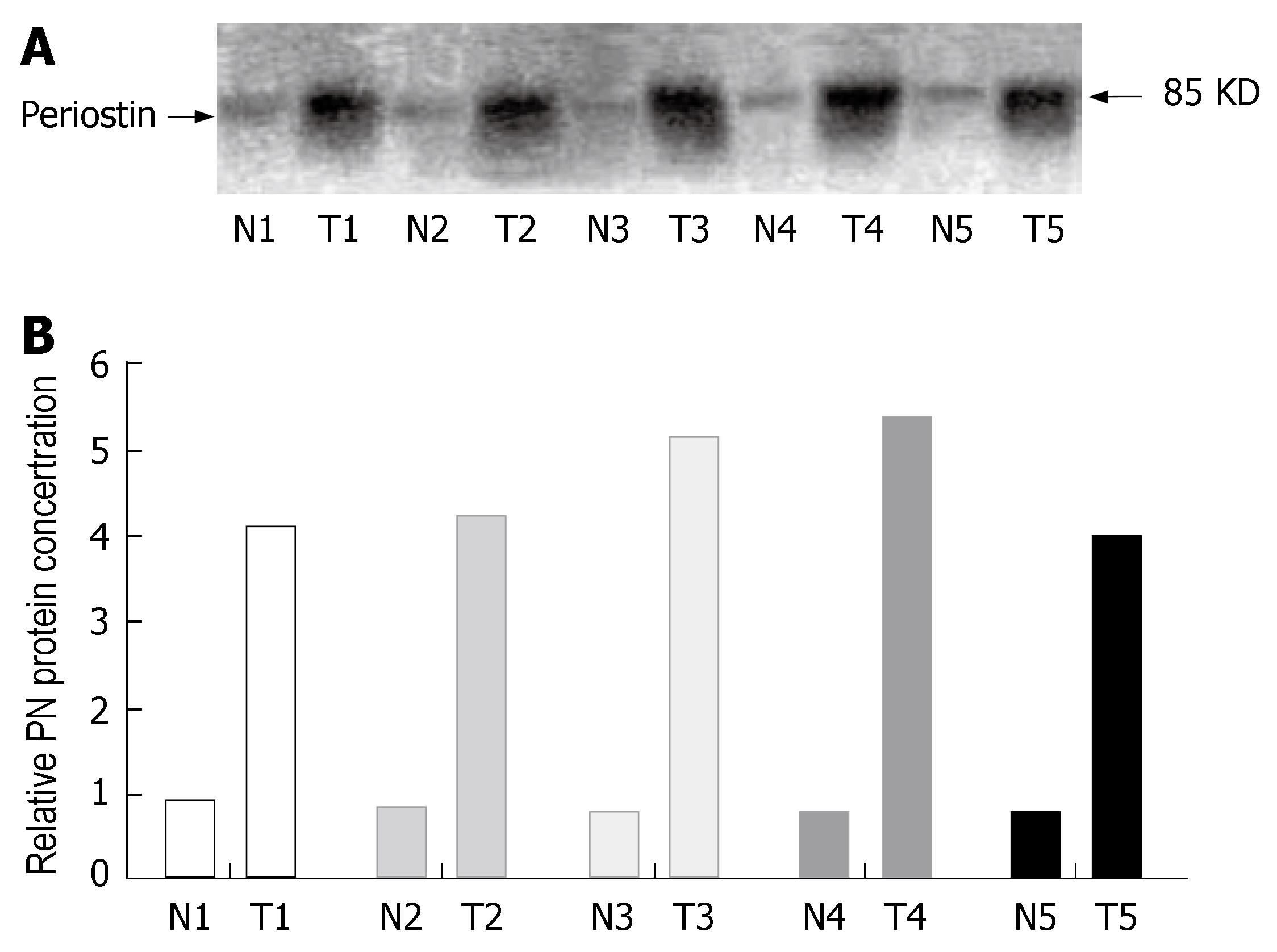
To determine if the higher level of periostin mRNA expression revealed by RT-PCR analysis was directly linked to increased levels of periostin protein expression, we performed Western blot analysis with protein extracts from matched tissue sections (from which the mRNA samples were extracted). As shown in Figure 2A, periostin was found to be highly expressed in gastric cancer tissues from five different patients (T), whereas only faint periostin expression was found in the normal gastric tissues, with a fivefold overexpression of periostin in cancer tissues compared with normal tissues (P < 0.05, the density ratio was 0.8354 vs 4.5773) (Figure 2B). Taken together, these data demonstrate that periostin is highly expressed at both mRNA and protein levels in gastric cancer tissues.
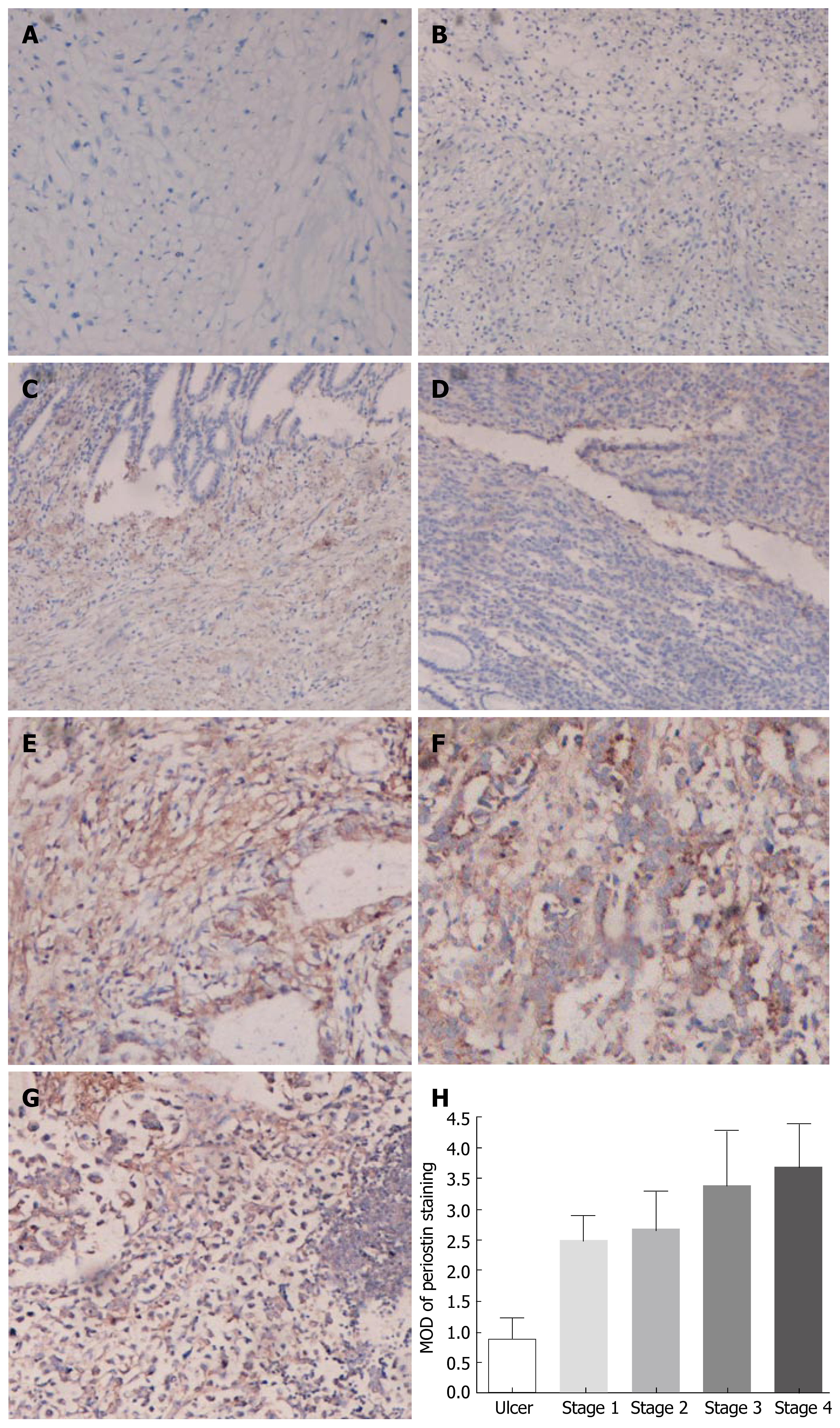
To investigate the significance of periostin overexpression in gastric cancer further, immunohistochemistry was carried out on five benign gastric ulcers and 35 cases of gastric cancer, which included: five stageItumors, eight stage II, 16 stage III, and six stage IV tumors, and five metastatic lymph nodes. As shown in Figure 3, the immunostaining indicated that high levels of periostin were present in the areas surrounding cancer cells, as well as in some cancer cells themselves. Periostin was also prominently stained in metastatic lymph nodes. In contrast, periostin expression was low in benign gastric ulcer tissues. Quantitative analysis of the periostin immunohistochemistry results indicated that the average MOD of periostin staining in the stageI-IV tumors was much higher than that in benign gastric ulcers in each group (P < 0.05). Furthermore, there was a trend for increasing periostin expression in stageI-IV tumors, although the difference was not statistically significant. In addition, there was no correlation between periostin expression and patient age and gender (data not shown). Taken together, these observations indicated that higher levels of periostin expression were associated with cancer progression and metastasis.
Many studies have already demonstrated that interactions between integrins of the tumor cell surface and adhesion molecules in the extracellular matrix (ECM) microenvironment may play a critical role in tumor cell migration, survival and growth[10-12]. The ECM-integrin interactions may also trigger intracellular signaling and activation of certain genes that leads to tumor cell proliferation during metastatic growth[13], and the formation of blood vessels within the tumor mass[14]. It is believed that integrins are also important in the attachment and metastasis of gastric cancer[8]. As a mesenchymal gene, periostin was formerly called osteoblast-specific factor-2, and was originally identified as an 811-amino acid protein secreted by osteoblasts[15]. It has structural homology with insect fasciclinIand can bind heparin and support adhesion of osteoblasts[16], which leads to the hypothesis that it functions to recruit and attach osteoblasts to the periosteum.
Periostin is upregulated in colorectal and breast cancer, and their liver metastases, which suggests that it plays a role in promoting growth in these tumors; furthermore, anti-periostin antibodies activate apoptosis and potentiate the effects of 5-fluorouracil chemotherapy in colorectal cancer[17,18]. Sasaki has reported[6] that periostin serum level is elevated in breast cancer with bone metastases, and has suggested it as a tumor marker in breast cancer. Since periostin functions as a ligand for integrins, and promotes ovarian cancer cell migration and adhesion[7], it is reasonable to investigate its role in gastric cancer.
In the present study, we found that periostin was expressed in normal gastric and gastric cancer tissues, while its expression was markedly elevated in cancer tissues. The basal mRNA and protein level of periostin in normal and benign gastric tissues suggest that it plays a role in the normal physiology of the gastrointestinal epithelium, as it was also found that periostin was expressed in normal colon tissue (data not shown). The dramatic increase in periostin in gastric cancer suggests its role in cancer progression. We found that periostin level was significantly increased in cancer tissues compared with gastric ulcers, and periostin level also increased with tumor-stage progression. As observed in other cancers, increased expression of periostin was associated with advanced stage and cell proliferation, adhesion and migration[5,19-27], and these results demonstrate that periostin may play a role in the progression of gastric cancer.
However, it has been reported that decreased expression of periostin is associated with progression of bladder cancer in humans, and expression of periostin mRNA is markedly reduced in a variety of human cancer cell lines[28,29]. These results differed from our findings that showed that periostin was upregulated in gastric cancer tissues at both the mRNA and protein level. We speculate that there are several explanations for the present findings. First, the expression profile of cancer cell lines may not reflect the in vivo expression pattern in certain tumors; second, periostin may have different functions according to different histopathological types of cancer; and third, it is possible that periostin may function differently by expressing alternative splicing events at the C-terminal region, as five different spliced transcripts of periostin are produced[15].
In summary, periostin expression was greater in gastric cancer tissues and metastatic lymph nodes compared to that in normal gastric tissues and benign gastric diseases (ulcers), and this increased expression was closely correlated with the TNM stage of gastric cancer. Our results strongly suggest that periostin plays a role in the progression of gastric cancer.
We thank the Department of Pathology, Zhong-Da hospital, Southeast University for providing all the paraffin blocks.
The pathogenesis of gastric cancer has been extensively investigated in recent years, and the expression of many genes changes in the progression of carcinogenesis, cell invasion and metastasis. Periostin was originally identified in a mouse osteoblastic library. Its role in tumorigenesis is still unclear.
Periostin has been suggested to be involved in cell adhesion and tumor formation. The human periostin gene has been shown to be overexpressed in lung cancer. Serum periostin levels are elevated in many kinds of human malignancy, and are correlated with poor prognosis. All of these observations suggest that periostin plays a role in tumorigenesis.
The expression of periostin is variable throughout the gastrointestinal tract. The basic level of this protein expression suggests that it plays a role in the normal physiology of the gastrointestinal epithelium. However, the expression of periostin mRNA differs between primary tumors and their respective cell lines. For example, periostin mRNA expression is low in colorectal cancer and head and neck squamous cell carcinoma cell lines, but higher in the primary tumors. The possibility is that stromal components play a role in stimulating periostin expression. Furthermore, opposite findings have been reported, periostin is down-regulated in bladder cancer, and invasiveness and metastasis are suppressed by periostin.
This study has implications for the future investigation of mesenchyme-specific genes in the formation of gastrointestinal cancer. Regarding the higher expression of periostin in gastric cancer, at both the mRNA and protein level, it is possible that periostin plays a role in the development of gastric cancer, and further study of this molecule in gastric cancer and its role as a biological marker is warranted.
This is a well-written study which investigated the expression of the periostin gene. There has not been much research on this molecule in gastric cancer, and this study revealed that periostin was more highly expressed at the mRNA and protein level in gastric cancer, and the expression level was positively correlated with clinical tumor stage. Further investigation regarding the function and prognostic significance of periostin will be interesting, and this paper is a valuable addition to the literature on gastric cancer.
S- Editor Liu Y L- Editor Kerr C E- Editor Li HY
| 1. | Chen J, Röcken C, Malfertheiner P, Ebert MP. Recent advances in molecular diagnosis and therapy of gastric cancer. Dig Dis. 2004;22:380-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Kountouras J, Zavos C, Chatzopoulos D. New concepts of molecular biology on gastric carcinogenesis. Hepatogastroenterology. 2005;52:1305-1312. [PubMed] [Cited in This Article: ] |
| 3. | Vajkoczy P, Menger MD, Goldbrunner R, Ge S, Fong TA, Vollmar B, Schilling L, Ullrich A, Hirth KP, Tonn JC. Targeting angiogenesis inhibits tumor infiltration and expression of the pro-invasive protein SPARC. Int J Cancer. 2000;87:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 4. | Lochter A, Werb Z, Bissell MJ. Transcriptional regulation of stromelysin-1 gene expression is altered during progression of mouse mammary epithelial cells from functionally normal to malignant. Matrix Biol. 1999;18:455-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358-5364. [PubMed] [Cited in This Article: ] |
| 6. | Sasaki H, Yu CY, Dai M, Tam C, Loda M, Auclair D, Chen LB, Elias A. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res Treat. 2003;77:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Ke JJ, Shao QS, Ling ZQ. Expression of E-selectin, integrin beta1 and immunoglobulin superfamily member in human gastric carcinoma cells and its clinicopathologic significance. World J Gastroenterol. 2006;12:3609-3611. [PubMed] [Cited in This Article: ] |
| 9. | Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350-7356. [PubMed] [Cited in This Article: ] |
| 10. | Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 792] [Cited by in F6Publishing: 773] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 11. | Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111:923-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1724] [Cited by in F6Publishing: 1678] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 13. | Meredith JE, Schwartz MA. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729-3738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 463] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271-278. [PubMed] [Cited in This Article: ] |
| 16. | Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 730] [Cited by in F6Publishing: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 17. | Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis. 2005;26:908-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992-4003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA, Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082-2094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, Miyauchi M, Takata T. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928-6935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77-83. [PubMed] [Cited in This Article: ] |
| 24. | Sasaki H, Sato Y, Kondo S, Fukai I, Kiriyama M, Yamakawa Y, Fuji Y. Expression of the periostin mRNA level in neuroblastoma. J Pediatr Surg. 2002;37:1293-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Sasaki H, Dai M, Auclair D, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y, Chen LB. Serum level of the periostin, a homologue of an insect cell adhesion molecule, in thymoma patients. Cancer Lett. 2001;172:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Sasaki H, Dai M, Auclair D, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y, Chen LB. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer. 2001;92:843-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 27. | Sasaki H, Lo KM, Chen LB, Auclair D, Nakashima Y, Moriyama S, Fukai I, Tam C, Loda M, Fujii Y. Expression of Periostin, homologous with an insect cell adhesion molecule, as a prognostic marker in non-small cell lung cancers. Jpn J Cancer Res. 2001;92:869-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H. Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int J Cancer. 2005;117:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Yoshioka N, Fuji S, Shimakage M, Kodama K, Hakura A, Yutsudo M, Inoue H, Nojima H. Suppression of anchorage-independent growth of human cancer cell lines by the TRIF52/periostin/OSF-2 gene. Exp Cell Res. 2002;279:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |