Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5217
Revised: July 12, 2007
Accepted: August 18, 2007
Published online: October 21, 2007
AIM: To test the hypothesis that hydrolysis of sphingomyelin to ceramide changes the composition of tight junctions (TJs) with increasing permeability of the intestinal epithelium.
METHODS: Monolayers of Caco-2 cells were used as an in vitro model for the intestinal barrier. Permeability was determined by quantification of transepithelial flux and transepithelial resistance. Sphingolipid-rich membrane microdomains were isolated by a discontinuous sucrose gradient and characterized by Western-blot. Lipid content of microdomains was analysed by tandem mass spectrometry. Ceramide was subcellularly localized by immunofluorescent staining.
RESULTS: Exogenous sphingomyelinase increased transepithelial permeability and decreased transepithelial resistance at concentrations as low as 0.01 U/mL. Lipid analysis showed rapid accumulation of ceramide in the membrane fractions containing occludin and claudin-4, representing TJs. In these fractions we observed a concomitant decrease of sphingomyelin and cholesterol with increasing concentrations of ceramide. Immunofluorescent staining confirmed clustering of ceramide at the sites of cell-cell contacts. Neutralization of surface ceramide prevented the permeability-increase induced by platelet activating factor.
CONCLUSION: Our findings indicate that changes in lipid composition of TJs impair epithelial barrier functions. Generation of ceramide by sphingomyelinases might contribute to disturbed barrier function seen in diseases such as inflammatory, infectious, toxic or radiogenic bowel disease.
- Citation: Bock J, Liebisch G, Schweimer J, Schmitz G, Rogler G. Exogenous sphingomyelinase causes impaired intestinal epithelial barrier function. World J Gastroenterol 2007; 13(39): 5217-5225
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5217
Abnormal mucosal permeability is a hallmark of inflammatory bowel disease (IBD)[1,2]. The epithelial barrier function and its relevance in IBD pathophysiology has gained increasing attention in recent years[2] as it may contribute to increased bacterial translocation and subsequent inflammatory responses in the mucosa.
Many signaling molecules involved in the pathogenesis of IBD such as TNF-α or IFN-γ may cause an alteration of the lipid composition in the cell membrane by activation of sphingomyelinases (SMases)[3,4]. SMases are characterized by a specific optimal pH and accordingly are divided into acid, neutral and basic sphingomyelinase species. The acid sphingomyelinase (ASM) contributes to lysosomal sphingomyelin turnover and is also secreted upon cellular treatment with inflammatory stimuli[5]. In contrast, neutral SMase is membrane-bound, alkaline SMase is found in the bile. Activation of SMases is followed by rapid hydrolysis of plasma membrane sphingomyelin to the second messenger ceramide[6]. The sphingolipid ceramide is an important messenger involved in many signaling pathways with influences on cell differentiation, growth suppression and apoptosis. It is generated upon ligation of receptors like cluster of differentiation (CD) 40, CD95, IL-1 or TNF receptor[7-10], as response to ionizing radiation[11-13], ischemia-reperfusion injury[14] and infections with bacteria like Neisseria gonorrhoeae and Pseudomonas aeruginosa[15-17] or viruses like Sindbis- or Rhinovirus[18,19]. Ceramide alters the composition of cholesterol- and sphingolipid-enriched membrane microdomains[20] and thereby promotes transmembrane signaling[7-9]. It also has the capacity to restructure the membrane to allow the release of vesicles[21].
Grassme et al[7,8] have demonstrated that cellular stimulation triggers a translocation of the acid sphingomyelinase from intracellular stores onto the extracellular leaflet of the cell membrane. Surface ASM initiates a release of ceramide which mediates clustering of sphingolipid-rich membrane domains, termed “lipid rafts”. Lipid rafts are also described as detergent insoluble cholesterol- and glycosphingolipid-enriched membrane microdomains (DIGs) because the tight packing of the lipids renders rafts resistant to solubilization by non-ionic detergents at low temperatures[22,23].
Intestinal permeability is influenced by the lipid content of epithelial cells. Dietary fatty acids are known to affect barrier function of the mucosa[24,25]. Clinical studies showed that omega 3-fatty acids may be of beneficial effect on the course of Crohns disease[26,27]. Paracellular permeability is controlled by a junctional complex of proteins and lipids which form different strands, commonly described as adherens-junction and tight-junction (TJ). TJs have been identified as microdomains in the plasma membrane with similar characteristics as DIGs[28,29]. Depletion of cholesterol from Caco-2 cell layers increases permeability[30], suggesting that cholesterol is critical in maintaining the barrier function.
The proinflammatory cytokines TNF-α and IFN-γ, known to induce ceramide-generation, have recently been shown to disrupt the barrier function of epithelial cells independent from their apoptosis-inducing property[31]. With regard to the finding that ceramide displaces cholesterol from sphingolipid-enriched microdomains[20], we hypothesized that formation of ceramide may be an initial event leading to structural lipid-rearrangements of TJs with impaired barrier integrity.
To test this hypothesis we used the intestinal epithelial cell line Caco-2 to assess the effect of exogenous SMase on barrier function and the accompanying lipid composition of TJs.
The human intestinal epithelial cell line Caco-2 was obtained from American Type Culture collection (HTB 37). Cells were maintained in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1% non-essential amino acids 1% sodium pyruvate in an atmosphere containing 10% CO2 at 37°C. Sphingomyelinase from staphylococcus aureus, platelet activating factor (PAF) and deoxycholic acid were purchased from Sigma-Aldrich, Germany.
The following antibodies were used for Western-blot analysis: Goat polyclonal anti-occludin (C-19) and goat polyclonal anti-claudin-4 (C-18) from Santa Cruz, CA, USA; mouse anti-E-cadherin (clone HECD-1) from Calbiochem, CA, USA; rabbit polyclonal anti p38 mitogen activated protein kinase (MAPK) from New England Biolabs, Beverly, MA, USA. The rabbit polyconal anti-toll like receptor 4 (TLR4) Ab was a kind gift from Dr. Werner Falk, Regensburg, Germany. For immunohistochemical staining of tight junctions we used the rabbit polyclonal anti-ZO-1 Ab from Zymed lab. Inc., CA, USA. For visualization and neutralization of ceramide, the monoclonal mouse IgM anti-Ceramide Ab (MID 15B4) from Alexis, Germany was used.
For permeability assays, Caco-2 cells were seeded in 12 well plates with a growth area of 1.0 cm2 and a pore size of 3 μm (Transwell® permeable supports by Corning Incorporated, MA, USA) at a density of 2 × 105 cells/cm2. Media was replaced every 3 or 4 d. Experiments were performed 13-15 d after cells reached confluency with a transepithelial electrical resistance (TEER) between 500-750 Ω∙cm2. Permeability was quantified by measuring the transepithelial flux of fluorescein-sulfonic acid (Molecular Probes Inc., Germany). After treatment of Caco-2 monolayers with the indicated substances, fluorescein-sulfonic acid was added to the apical side of the monolayers at a final concentration of 100 μg/mL. After incubation for 4 h 100 μL aliquots of medium were removed from the basolateral chambers, and fluorescence signal by fluorescein was measured using a fluorescence microplate reader. TEER of the Caco-2 monolayers was measured using a Millicell®-ERS epithelial voltohmmeter by Millipore with a pair of chopstick electrodes. Untreated monolayers were used as negative controls. All measurements were performed in duplicate.
Caco-2 cells were incubated with the indicated substances 6 h before measurement of caspase-activity. As a positive control for induction of apoptosis, cells were treated with deoxycholic acid (DCA) for 1 h. DCA was sonicated at 40°C for 20 min prior to the experiments. The colorimetric caspase-3/7-activity assay Apo-ONETM (Promega, WI, USA) was used according to the manufacturer's recommendations. Activity was quantified using a fluorescence microplate reader with appropriate wavelengths for excitation (485 nm) and emission (530 nm).
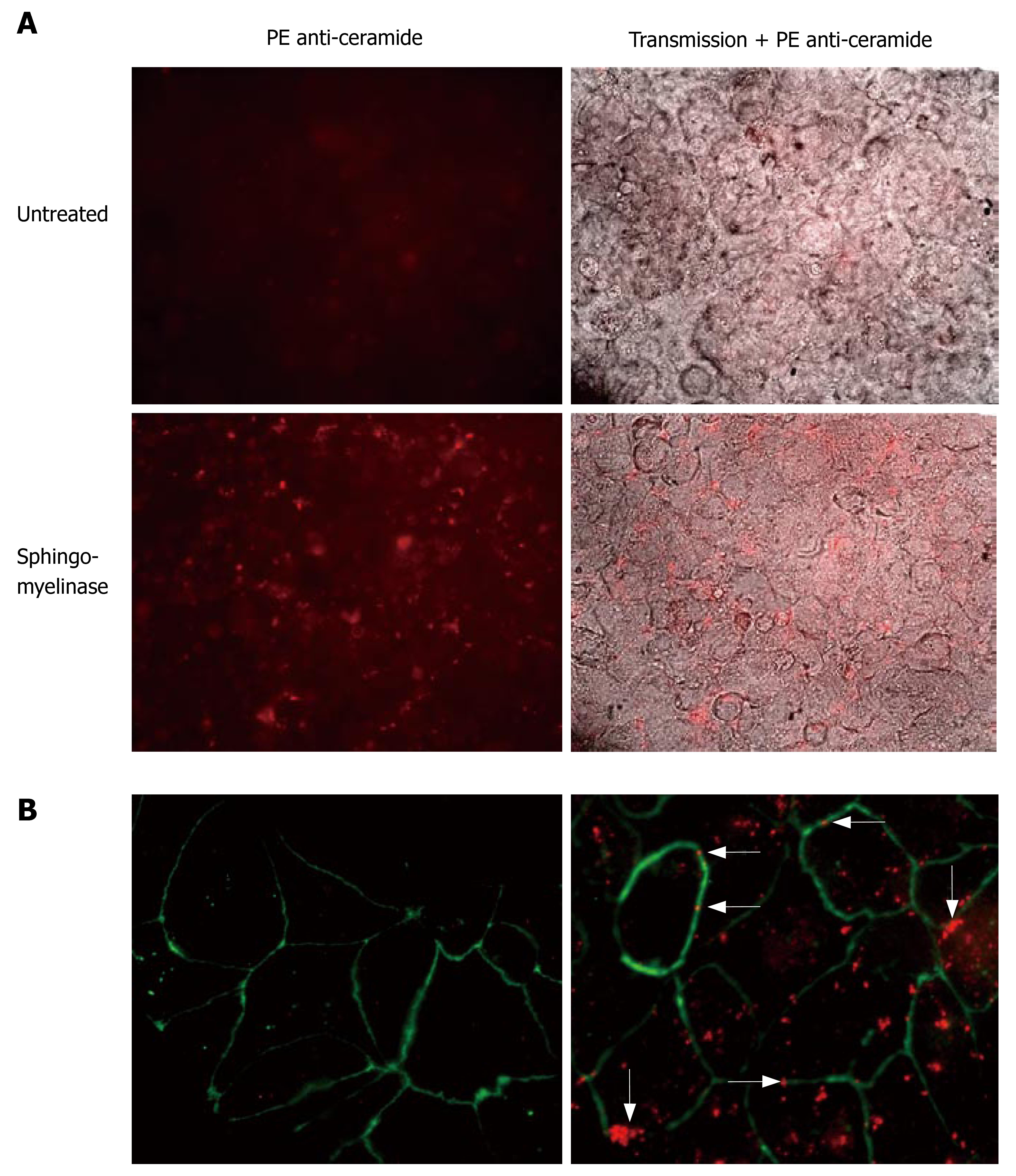
For fluorescence studies Caco-2 cells were grown on chamber slides (Nunc, Germany) and stimulated with 0.25 U/mL SMase for 10 min. After stimulation the cells were washed with phosphate buffered saline (PBS) at 4°C and fixed for 15 min in 1 % (w/v) paraformaldehyde in PBS at room temperature. Then cells were washed three times with PBS and blocked with Tween 20 0.05% in PBS. Cells were washed again and then incubated with anti-ceramide mAb 15B4 (1/50 dilution) and anti ZO-1 Ab for 30 min in washing buffer (PBS with 2% fetal calf serum, 0.01% NaN3) to block sites of non-specific binding. The anti-ceramide Ab was visualized with a PE-labeled anti-mouse IgM, the anti-ZO-1 Ab with a fluorescein-isothiocyanate (FITC)-anti rabbit Ab. Fluorescence staining was viewed with an Axiovert 100 fluorescence microscope (Zeiss, Germany).
Confluent layers of Caco-2 cells were stimulated in 75 cm2 flasks in 5 mL of culture media. Stimulation was terminated by washing the cells with 5 mL ice-cold TNE (25 mmol/L Tris–HCl, pH 7.4, 150 mmol/L NaCl, and 5 mmol/L EDTA) on ice. Cells were washed twice with TNE at 4°C and lysed for 20 min in 1.5 mL of ice-cold TNE containing 1% of Triton X-100 and protease inhibitors (10 μg of aprotinin and leupeptin and 200 μmol/L phenylmethylsulfonyl fluoride (PMSF). Cells were then further homogenized with 20 strokes in a Wheaton loose fitting Dounce-homogenizer. Nuclei and cellular debris were pelleted by centrifugation at 600 g for 5 min, 4°C. To isolate low-density, Triton X-100-insoluble complexes, the supernatant was adjusted to 40% sucrose, transferred to an ultracentrifugation tube, and overlaid with a 35% and 5% discontinuous sucrose gradient. Ultracentrifugation was performed at 29000 r/min (110000 ×g) at 4°C for 16 h in a Beckman SW41 rotor. Gradient fractions (1 mL) were either collected from the top of the tube or from the 35%/5%-interface to compare the effect of cell stimulation on raft fractions. For mass spectrometry, fractions were prepared as described below. For Western blotting, proteins were precipitated using 400 μL of 10% trichloroacetic acid, neutralized and separated by 10% SDS-PAGE.
Proteins were separated by 10% SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane (Hybond). Blots were blocked with 5% nonfat dry milk powder and incubated overnight at 4°C with the indicated primary antibodies. All antibodies were diluted 1:750 in Tris-buffered saline supplemented with 0.1% Tween 20. The membranes were further incubated with peroxidase-conjugated secondary antibodies, and protein bands were visualized using a commercial chemiluminescence detection kit (ECL plus, Amersham Biosciences).
Lipids were quantified by electrospray ionization tandem mass spectrometry (ESI-MS/MS) in positive ion mode as described previously[32-35]. Samples were quantified by direct flow injection analysis using the analytical setup and the data analysis algorithms described by Liebisch et al[34]. A parent ion scan of m/z 184 specific for phosphocholine-containing lipids was used for phosphatidylcholine, sphingomyelin[33] and lysophosphatidylcholine[33]. Neutral loss scans of m/z 141 and m/z 185 were used for phosphatidylethanolamine and phosphatidylserine, respectively. Ceramide was analyzed similar to a previously described method[35] using N-heptanoyl-sphingosine as internal standard. Free cholesterol and cholesteryl esters were quantified using a fragment ion of m/z 369 after selective derivatization of free cholesterol[36]. Quantification was achieved by calibration lines generated by addition of naturally occurring lipid species to cell homogenates[32-36].
Data are shown using vertical scatter plots with Box-Whisker plots (25% and 75% values), generated in the basic module of the program SigmaPlot. Statistical analysis was performed by Mann-Whitney U-test, with P < 0.05 considered statistically significant. Data are given as means ± SE (SD in case of lipid analysis).
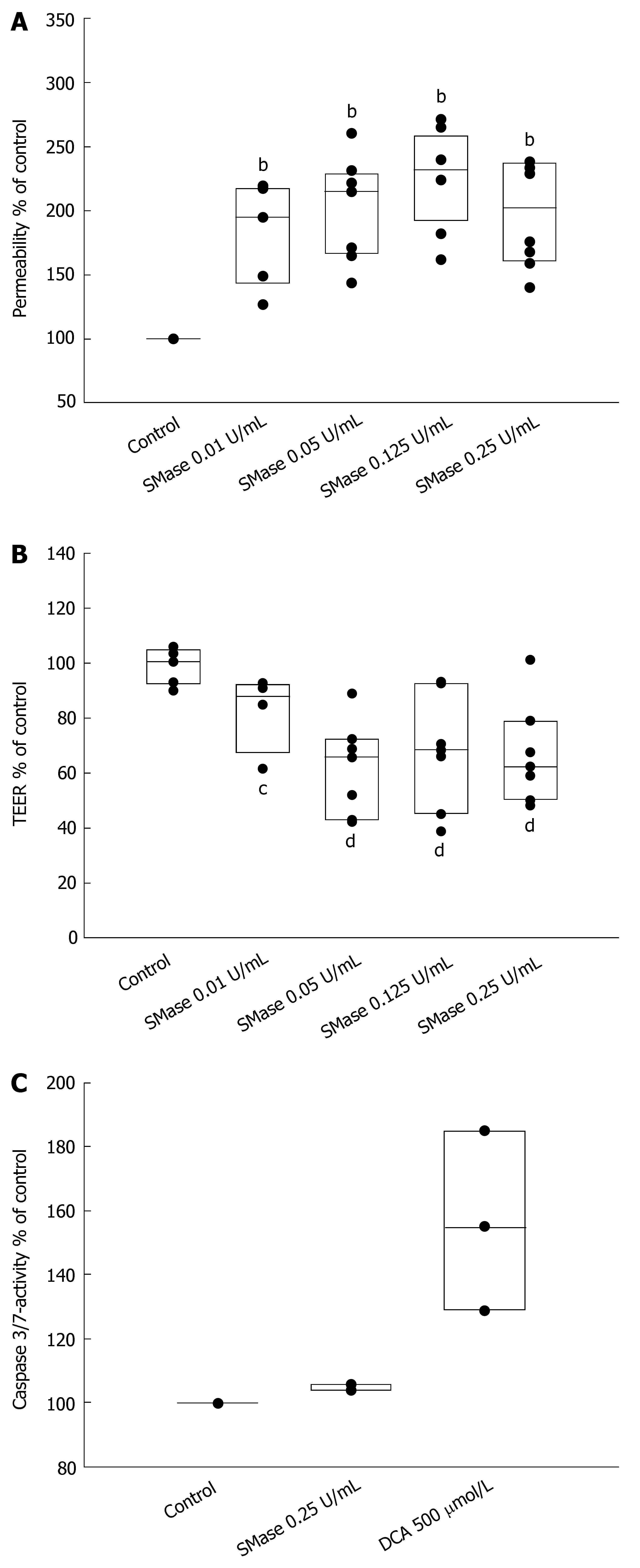
To study a potential regulation of intestinal permeability by sphingomyelinases, Caco-2 cell layers were exposed to different concentrations of exogenous SMase. Transepithelial permeability was determined by measurement of transepithelial flux of fluorescein-sulfonic acid across a monolayer grown on permeable supports. Incubation with SMase to the apical chamber induced a concentration-dependent increase of permeability which could be detected at concentrations as low as 0.01 U/mL SMase (181.6% ± 16.7%, P < 0.01) (Figure 1A). Using 0.05 U/mL SMase, permeability was increased by 201.1% ± 15.8% (P < 0.01) and by 224.0% ± 18.0% (P < 0.01) when 0.125 U/mL SMase were used. Increase of SMase-concentration to 0.25 U/mL did not further increase transepithelial flux (192.0% ± 15.3%, P < 0.01) (Figure 1A). In a different set of experiments with the same experimental conditions, PAF was used as a positive control. At a concentration of 5 μmol/L, PAF increased permeability by 162.8% ± 13.0% (Figure 2).
To gain insight into the mechanisms of ceramide-mediated permeability we measured the transepithelial electrical resistance (TEER). Exogenous SMase produced a significant decline in TEER at concentrations as low as 0.01 U/mL (17.5% ± 6.2%, P < 0.05) (Figure 1B). The fall in TEER with 0.05 U/mL was much higher (38.1% ± 6.0%, P < 0.01). Using 0.125 U/mL SMase or 0.25 U/mL SMase did not further decrease TEER (32.2% ± 7.3%, P < 0.01 and 33.2% ± 6.4%, P < 0.01, respectively).
To exclude apoptotic or necrotic cell death caused by SMase within the time frame of our experiments, caspase-3/7-activity and LDH release assays were performed. As shown in Figure 1C, 0.25 U/mL SMase induced no activation of caspase-3/7 within 6 h. Deoxycholic acid (500 μmol/L for 1 h) was used as a positive control. Release of LDH from Caco-2 monolayers by SMase was also not detectable (data not shown).
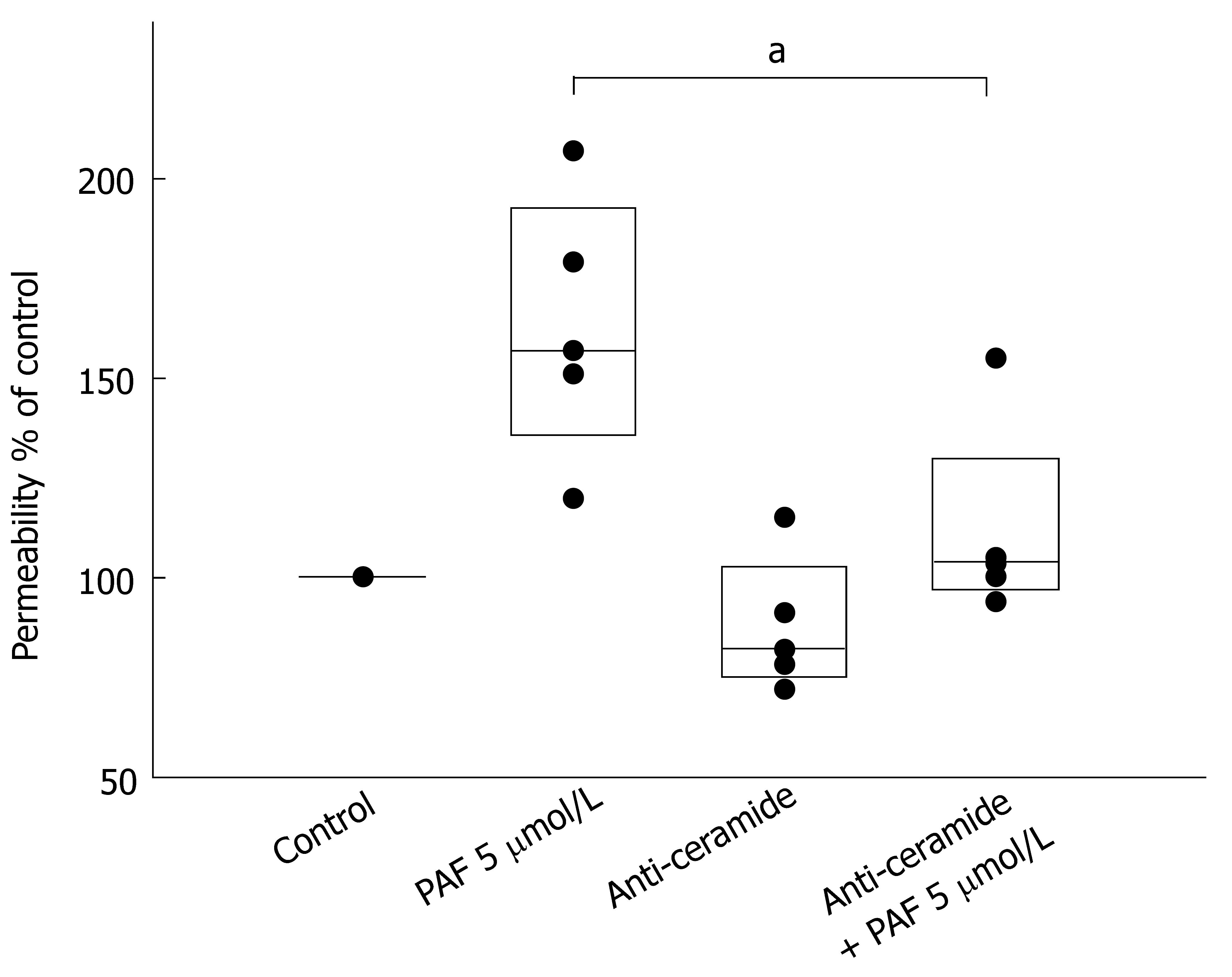
Next, we investigated whether the increased permeability induced by PAF might be linked to rearrangement of tight-junctional lipids. Incubation of the monolayers with 5 μmol/L PAF increased permeability by 162.8% ± 13.0% (Figure 2). To examine the role of ceramide in PAF-mediated permeability we co-incubated Caco-2 cell layers with ceramide-antiserum (1/100 dilution). Co-incubation of the Caco-2-monolayer with ceramide-antiserum prevented the increase of permeability induced by 5 μmol/L PAF (111.6% ± 9.86%, P < 0.05) (Figure 2), indicating a stabilization of tight-junctional complexes by the IgM-anti-ceramide Abs.
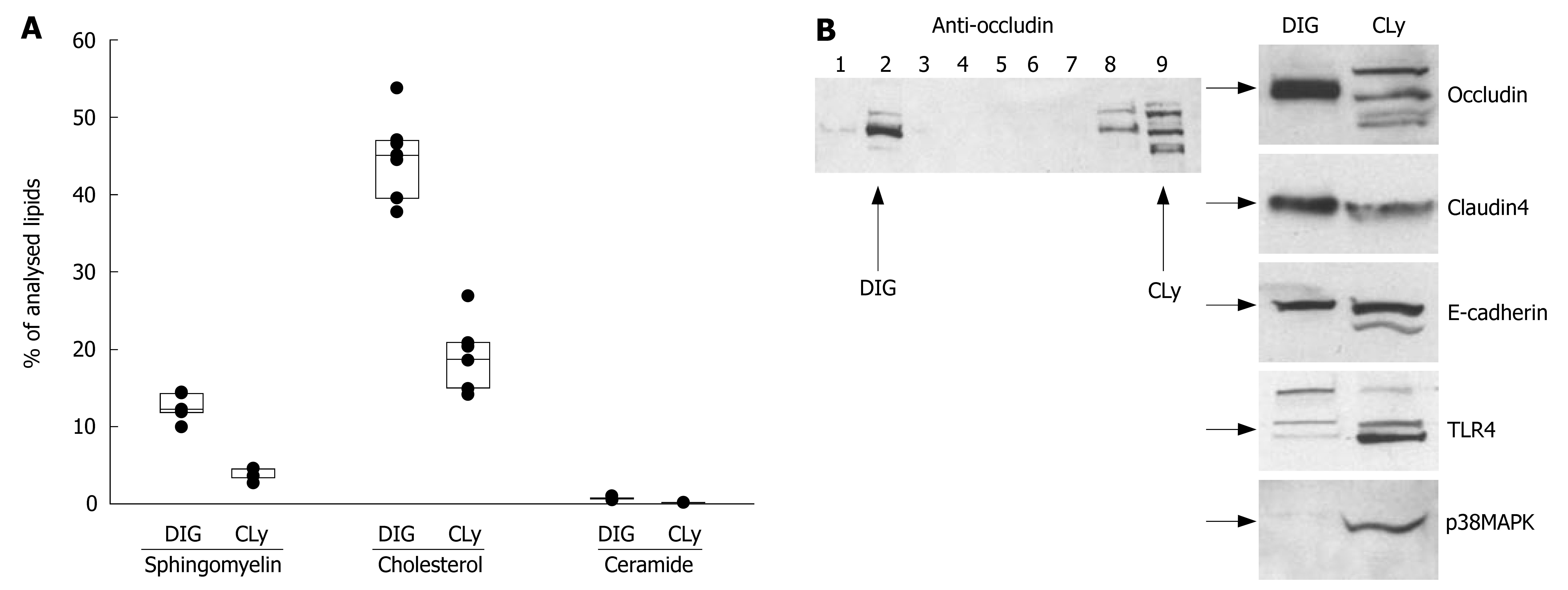
To further test our hypothesis, DIGs were isolated using sucrose gradient techniques and analysed for their lipid- and protein-composition. Analysis of the lipid composition by tandem mass spectrometry revealed the presence of high amounts of sphingomyelin (12.5% ± 1.4%), cholesterol (44.9% ± 4.9%) and ceramide (0.74% ± 0.18%) in DIGs with only 3.8% ± 0.6%, 19.2% ± 3.9% and 0.19% ± 0.03% in the total cell lysate (including DIGs), respectively (Figure 3A, values are given as % of analysed lipids ± SD). This indicated a good separation of DIGs from other membrane components. To ascertain isolation of tight junctions within the Triton X-100 insoluble preparations, Western-blot experiments were performed to prove the presence of tight-junctional proteins. As shown in Figure 3B, major pools of the tight-junctional proteins occludin and claudin-4 were present in Triton X-100 insoluble preparations and to a lesser extent in cell lysate. The basolateral membrane protein E-cadherin was also present in DIGs but was not as prominent. To exclude contamination with proteins not associated with DIGs, Western-blots of TLR4 and p38MAPK were performed which could not be detected in preparations of DIGs (Figure 3B).
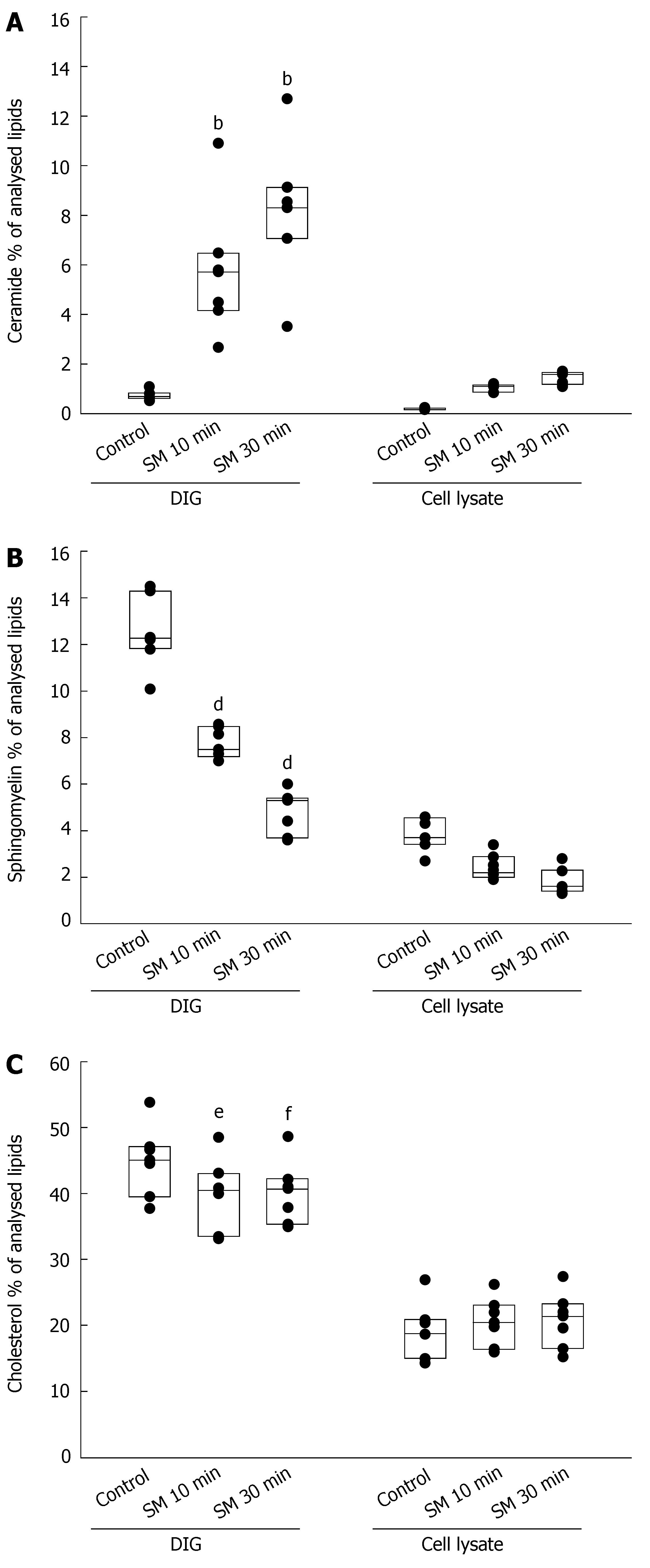
Tandem mass spectrometry was used to analyze the effect of SMase on the lipid content of DIGs which overlap with tight junctions. Incubation of Caco-2 monolayers with 0.25 U/mL SMase resulted in rapid increase of ceramide in DIGs by 5.0% ± 2.4% after 10 min (P < 0.01) and 7.3% ± 2.6% after 30 min (P < 0.01), whereas the increase was only 0.84% ± 0.14% and 1.25% ± 0.23% in the whole cell lysate (including DIGs), respectively (Figure 4A, data shown as % of analysed lipids). In the same preparations of DIGs the concentration of sphingomyelin decreased by 4.73% ± 1.4% after 10 min (P < 0.01) and 7.6% ± 2.2% after 30 min (P < 0.01) (Figure 4B). The concentration of cholesterol declined by 5.0% ± 3.2% after 10 min and 4.8% ± 2.3% after 30 min (Figure 4C). This reflects a decrease of the percentage of cholesterol by 10.9% ± 7.3% after 10 min (P < 0.05) and 10.6% ± 4.8% after 30 min (P < 0.01).
Fluorescence microscopy was performed to demonstrate the localization of ceramide accumulation. We induced generation of ceramide by incubation of Caco-2 cells with 0.25 U/mL SMase for 10 min. After stimulation, staining of the cells with anti-ceramide 15B4 antibodies revealed the formation of ceramide-clusters (Figure 5A and B) that were frequently located at the sites of cell-cell-contact. Costaining for the tight-junction protein ZO-1 confirmed colocalization of ceramide-clusters with the junctional complexes (Figure 5B).
These data indicate that the generation of ceramide by SMase leads to localized accumulation of ceramide at the sites of cell/cell-contact with increased intestinal permeability and suggest a central role of ceramide in the regulation of barrier integrity.
Our data indicate that hydrolysis of sphingomyelin to ceramide increases intestinal epithelial cell permeability in a well-established model of Caco-2 cell monolayers[37]. To obtain naturally occurring long chain ceramides, formation of ceramide was induced by addition of bacterial sphingomyelinase (SMase). This approach was used because neutral as well as acid SMase are capable of generating ceramide in the outer leaflet of the cell membrane by rapid hydrolysis of membrane sphingomyelin. Previous studies suggested that, upon cellular stimulation, the acid sphingomyelinase (ASM) translocates onto the outer surface of sphingolipid-rich membrane microdomains[7,8]. The fact that ASM activity is also increased in the serum of mice treated with endotoxin[38] or PAF[39], indicates a possible role of extracellularly located ASM. Another advantage of using exogenous SMase is the enzymatic cleavage of sphingomyelin at the sites of sphingomyelin-enriched domains in the cell membrane, whereas the addition of long chain ceramides might lead to lateral assembly of ceramide to pre-existing DIGs (and/or TJs) without affecting the structure inside these microdomains. Apart from that, exogenous ceramides are likely to be rapidly metabolised, thus having a rather transient effect on lipid composition. Exogenous SMase was effective in terms of increased permeability and decrease of TEER, indicating a reduction of the paracellular barrier by hydrolysis of sphingomyelin. Other possible effects of SMase include an increase of transcellular transport of vesicles. Therefore, we measured intracellular fluorescence after exposure of the cells with SMase and fluorescein-sulfonic acid with colorimetric assays of the cell lysates and performed transepithelial flux experiments with simultaneous colchicine-treatment (data not shown). In both cases we did not observe any differences, making transcellular transport unlikely. To exclude damage of monolayers as a possible cause for increased permeability, activity of caspase-3/7 in the cell lysate and lactate dehydrogenase (LDH) in the supernatant were measured without significant results.
Lipid analysis of Triton X-100 insoluble preparations clearly demonstrated the separation of membrane portions which were enriched in cholesterol, sphingomyelin and ceramide. Western-blot experiments of DIGs revealed that these preparations contained major pools of the TJ-proteins occludin and claudin-4. Upon stimulation with SMase we detected a rapid accumulation of ceramide in the same membrane fractions, indicating selective accumulation of ceramide in sphingolipid-enriched membrane microdomains, including junctional complexes.
Previous studies suggested displacement of cholesterol from DIGs by ceramide[20,40,41]. To our knowledge, there is no data in the available literature about the modulation of ceramide and cholesterol in TJs by sphingomyelinases. Therefore we compared the content of cholesterol in DIGs which overlap with TJs. In accordance with these studies we also observed a decrease of cholesterol in these microdomains with increasing concentrations of ceramide, suggesting a rearrangement of tight junctional lipids by ceramide with impaired barrier function.
Lambert et al[30] showed a decrease of TEER by 80%-90% after extraction of 40%-45% of cholesterol from Caco-2 monolayers. Compared to these results the decrease of tight-junctional cholesterol by ceramide is moderate. Nevertheless, these changes could explain early disturbances of tight-junctional integrity upon cellular stress, deriving from toxic, infectious or immunologic challenges of the intestinal epithelium. Therefore, we propose the following pathophysiologic model: Cellular stimulation leads to localized accumulation of ceramide with concomitant decrease of sphingomyelin and cholesterol leading to destabilization of tight junctional strands and loss of barrier integrity.
Long chain ceramides also have the unique property of fusing membrane domains and tend to form cluster, which was also observed in our studies. Mechanisms of ceramide to modify the structure of bilayers and their effect in promoting efflux and release have been studied in several models[21,42,43]. Montes et al[21] demonstrated release of molecules with a molecular mass up to 20 kDa upon treatment of unilamellar vesicles with sphingomyelinase. A possible mechanism for this release was suggested by Siskind and Colombini who detected formation of large stable channels by ceramide in planar bilayers through electrophysiological methods[43]. The influence of ceramide on the architecture of TJs also seems to be important in the PAF-mediated increase of permeability. Pretreatment of Caco-2 cells with a monoclonal IgM ceramide-antiserum prevented the loss of barrier function induced by 5 μmol/L PAF.
Together, these findings provide evidence for a new aspect of cellular ceramide generation. Lipid rearrangements triggered by enzymatically catalysed formation of ceramide upon cellular stimulation may be a frequent process for localized exposure of intraluminal antigens to the immune system. Repeated stimulation or extension of the stimulated area may lead to an imbalance of the lipid geometry producing a stronger leakiness of the affected junctional complexes resulting in initiation of an inflammatory process which may further perpetuate itself. Better knowledge of the relevant lipids which control this “lipid-barrier” and their modifying enzymes would be helpful to develop treatment strategies to strengthen this barrier. Lipid-enriched diets or inhibition of relevant lipid-modifying enzymes could be possible treatment modalities to control chronic inflammation of the intestine as seen in IBD[44].
In summary, our data demonstrate that hydrolysis of sphingomyelin to ceramide affects transepithelial permeability and resistance. Long chain ceramides, generated by SMase, accumulate in cholesterol- and sphingolipid-enriched membrane microdomains which include TJs. These microdomains fuse to large, ceramide-enriched clusters, located on the surface of the cells and at the sites of cell-cell contact, explaining the effect of long-chain ceramides on barrier integrity. Finally, the effect of PAF on paracellular permeability is inhibited by neutralization of surface ceramide, suggesting a regulation of the paracellular permeability by the arrangement of membrane ceramide.
The sphingolipid ceramide, generated by signal-activated sphingomyelinases, has emerged as a second messenger of stimuli as diverse as ligation of various receptors, ionizing radiation, chemotherapy or infection with some bacteria and viruses. Upon stimulation of sphingomyelinases, ceramide is generated in distinct sphingolipid-enriched membrane microdomains of the cell membrane, termed “lipid rafts”. Tight junctions (TJs) are structurally related to lipid rafts, and thus, we hypothesized that hydrolysis of sphingomyelin to ceramide changes the composition of TJs with increasing permeability of the intestinal epithelium.
Intestinal permeability is influenced by the lipid content of epithelial cells and some lipids may be beneficial for the course of inflammatory bowel disease. Modification of the lipid content of TJs may be a possible strategy to improve intestinal barrier functions.
Our data indicate that hydrolysis of sphingomyelin to ceramide by sphingomyelinase (SMase) increases intestinal epithelial cell permeability. Lipid analysis after stimulation with SMase demonstrated rapid accumulation of ceramide in the membrane fractions which contain the TJ-proteins occludin and claudin-4, while sphingomyelin and cholesterol decrease. Pretreatment of cells with a monoclonal IgM ceramide-antiserum prevented the loss of barrier function induced by platelet activating factor.
Better knowledge of the relevant lipids and their modifying enzymes that control this “lipid-barrier” of the intestine would be helpful to develop treatment strategies to strengthen this barrier. Lipid-enriched diets or inhibition of relevant lipid-modifying enzymes could be possible treatment modalities to control chronic inflammation of the intestine as seen in inflammatory bowel disease.
Sphingomyelinase is an enzyme which hydrolyses sphingomyelin to ceramide upon various cellular stimuli. Paracellular permeability is controlled by a junctional complex of proteins and lipids which form different strands, commonly described as adherens-junction and tight-junction (TJ). Platelet activating factor is a lipid messenger which increases paracellular permeability in intestinal epithelial cells.
The authors set out to test the hypothesis that hydrolysis of sphingomyelin to ceramide changes the composition of tight junctions (TJs) with increasing permeability of the intestinal epithelium. Their findings suggested that changes in lipid composition of TJs impair epithelial barrier functions. Generation of ceramide by sphingomyelinases might contribute to disturbed barrier function seen in diseases such as inflammatory, infectious, toxic or radiogenic bowel disease.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Lu W
| 1. | Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Playford RJ, Ghosh S. Cytokines and growth factor modulators in intestinal inflammation and repair. J Pathol. 2005;205:417-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484-489. [PubMed] [Cited in This Article: ] |
| 4. | Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992;71:765-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 806] [Cited by in F6Publishing: 864] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 5. | Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem. 1998;273:18250-18259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070-7077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 328] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 7. | Grassmé H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168:298-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589-20596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 464] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 9. | Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954-23961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 313] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | García-Ruiz C, Colell A, Marí M, Morales A, Calvo M, Enrich C, Fernández-Checa JC. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Santana P, Peña LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 595] [Cited by in F6Publishing: 587] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 428] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1192] [Cited by in F6Publishing: 1129] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 14. | Yu ZF, Nikolova-Karakashian M, Zhou D, Cheng G, Schuchman EH, Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15:85-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Grassmé H, Gulbins E, Brenner B, Ferlinz K, Sandhoff K, Harzer K, Lang F, Meyer TF. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Hauck CR, Grassmé H, Bock J, Jendrossek V, Ferlinz K, Meyer TF, Gulbins E. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 2000;478:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Grassmé H, Jendrossek V, Riehle A, von Kürthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 412] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 18. | Jan JT, Chatterjee S, Griffin DE. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J Virol. 2000;74:6425-6432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Grassmé H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256-26262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Megha E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997-10004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 321] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Montes LR, Ruiz-Argüello MB, Goñi FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J Biol Chem. 2002;277:11788-11794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221-17224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1853] [Cited by in F6Publishing: 1754] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 23. | Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7533] [Cited by in F6Publishing: 7182] [Article Influence: 266.0] [Reference Citation Analysis (0)] |
| 24. | Rosella O, Sinclair A, Gibson PR. Polyunsaturated fatty acids reduce non-receptor-mediated transcellular permeation of protein across a model of intestinal epithelium in vitro. J Gastroenterol Hepatol. 2000;15:626-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Usami M, Komurasaki T, Hanada A, Kinoshita K, Ohata A. Effect of gamma-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition. 2003;19:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Shoda R, Matsueda K, Yamato S, Umeda N. Therapeutic efficacy of N-3 polyunsaturated fatty acid in experimental Crohn's disease. J Gastroenterol. 1995;30 Suppl 8:98-101. [PubMed] [Cited in This Article: ] |
| 27. | Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 559] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 28. | Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771-1781. [PubMed] [Cited in This Article: ] |
| 29. | Leung LW, Contreras RG, Flores-Maldonado C, Cereijido M, Rodriguez-Boulan E. Inhibitors of glycosphingolipid biosynthesis reduce transepithelial electrical resistance in MDCK I and FRT cells. Am J Physiol Cell Physiol. 2003;284:C1021-C1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Lambert D, O'Neill CA, Padfield PJ. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem J. 2005;387:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 634] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 32. | Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci USA. 1997;94:2339-2344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 726] [Cited by in F6Publishing: 740] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 33. | Liebisch G, Drobnik W, Lieser B, Schmitz G. High-throughput quantification of lysophosphatidylcholine by electrospray ionization tandem mass spectrometry. Clin Chem. 2002;48:2217-2224. [PubMed] [Cited in This Article: ] |
| 34. | Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim Biophys Acta. 2004;1686:108-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Liebisch G, Drobnik W, Reil M, Trümbach B, Arnecke R, Olgemöller B, Roscher A, Schmitz G. Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). J Lipid Res. 1999;40:1539-1546. [PubMed] [Cited in This Article: ] |
| 36. | Liebisch G, Binder M, Schifferer R, Langmann T, Schulz B, Schmitz G. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS). Biochim Biophys Acta. 2006;1761:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 37. | Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736-749. [PubMed] [Cited in This Article: ] |
| 38. | Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci USA. 2000;97:8681-8686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Göggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schütze S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 40. | Holopainen JM, Subramanian M, Kinnunen PK. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562-17570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Nurminen TA, Holopainen JM, Zhao H, Kinnunen PK. Observation of topical catalysis by sphingomyelinase coupled to microspheres. J Am Chem Soc. 2002;124:12129-12134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Ruiz-Argüello MB, Basáñez G, Goñi FM, Alonso A. Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J Biol Chem. 1996;271:26616-26621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640-38644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 44. | Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54:966-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |