Published online Jul 21, 2007. doi: 10.3748/wjg.v13.i27.3726
Revised: December 13, 2006
Accepted: December 18, 2006
Published online: July 21, 2007
AIM: To investigate the correlation between ezrin expression and types of gastric carcinoma and clinico-pathological variables.
METHODS: We examined ezrin protein expression in 75 gastric carcinoma (53 intestinal types of adenocarcinoma, 22 diffuse types of carcinoma) tissues by immunohistochemistry. The results were compared with clinicopathological parameters such as tumor type, grade of tumor, clinical stage, presence of metastatic lymph node, and depth of invasion.
RESULTS: Ezrin immunostaining was positive in 43 cases (81.1%) of intestinal type and in 9 (40.9%) cases of diffuse type adenocarcinomas (P < 0.001). In gastric carcinomas, the expression of ezrin protein correlated with the status of H pylori and survival. There was no correlation between expression of ezrin with TNM stage and histological grade of gastric carcinomas (P > 0.05).
CONCLUSION: The low expression of ezrin implicates the loss of adhesion in diffuse carcinomas. Furthermore, overexpression of ezrin in carcinomas with H pylori infection may be a genuine specific pathway in which H pylori may cause/initiate gastric carcinoma.
- Citation: Bal N, Yildirim S, Nursal TZ, Bolat F, Kayaselçuk F. Association of ezrin expression in intestinal and diffuse gastric carcinoma with clinicopathological parameters and tumor type. World J Gastroenterol 2007; 13(27): 3726-3729
- URL: https://www.wjgnet.com/1007-9327/full/v13/i27/3726.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i27.3726
Ezrin, a member of the ezrin-radixin-moesin (ERM) family of species-conserved protein in the band 4.1 superfamily, is a membrane cytoskeleton linker and is involved in cellular functions, including epithelial cell morphogenesis and adhesion. The proteins ezrin, moesin, and radixin act as linkers between the plasma membrane and the actin cytoskeleton. The inactivation of ezrin causes a massive cell retraction and leads to the destruction of both cell-cell and cell-substrate adhesion, whereas the overexpression of ezrin in insect cells results in enhanced cell adhesion[1-3]. Pujuguet et al[4] showed that ezrin plays a role in the transition from polarized epithelial cell form to a more spreading form by regulating the transport of E-cadherin to the plasma membrane. This mechanism between ezrin and E-cadherin may be important in its emerging role in tumor progression.
ERM proteins have a cytoskeletal linking function to connect the actin filaments to cell adhesion molecules such as CD43, CD44, ICAM 1, ICAM 2, modulating cell morphology, motility and adhesion[5]. The binding of ezrin to cell surface adhesion molecules is involved in cell migration and metastasis, because high levels of CD44 are associated with invasive and potentially metastatic tumor cells[5]. Ezrin is expressed at high levels in small intestine, stomach, pancreas and kidney, at intermediate levels in spleen, thymus, lymph nodes, at low levels in heart, brain and testis. In muscle and liver, expression of ezrin is not detected[6].
Human gastric carcinomas have been classified by Lauren into two major groups, intestinal (well differantiated) and diffuse (poorly differentiated) types. Intestinal type adenocarcinoma has better prognosis than diffuse type[7]. In the diffuse carcinoma, tumor cells are scattered in a fibrous stroma with loss of tight intracellular adhesions between cancer cells[7]. Although histopathological diagnosis is valuable in clinical medicine, the Lauren classification has been widely used because it separates two biological entities that are different in epidemiology, pathogenesis, behavior and carcinogenesis[8].
With the advent of new molecular technologies, information about grade of malignancy, prognosis and differential diagnosis can now be obtained[8-10]. Although ezrin is detected in many malignant cell types, such as adenocarcinoma of pancreas, renal cell carcinoma, and osteosarcoma, to the best of our knowledge we could not identify any information regarding ezrin and gastric carcinoma[5,6,11,12]. In this study, we aimed to document the relation of ezrin expression to the clinical outcome and the histological parameters in carcinomas of stomach.
For immunohistochemical analysis, 53 intestinal type adenocarcinoma and 22 diffuse type carcinoma tissue samples were selected from pathological archives. All patients with gastric carcinoma were treated surgically, diagnosed histologically, and followed up in the Department of General Surgery. Surgical staging was determined in accordance with the TNM criteria, 10 (13.3%) cases were classified as stage 1, 9 (12%) as stage 2, 23 (30.7%) as stage 3, and 33 (44.0%) as stage 4. The histological grade of each gastric cancer sample was determined according to the accepted criteria, resulting in 4 cases of gradeI(well differentiated), 25 grade II (moderately differentiated) and 46 grade III (poorly differentiated).
The samples were fixed in 10% neutrally buffered formalin and paraffin embedded. For immuno-histochemistry, serial sections of 5 μm thickness were cut from the paraffin blocks. The sections were deparaffinized with xylene and rehydrated with ethanol. Non-enzymatic antigen retrieval was performed on each slide and washed with phosphate-buffered saline (PBS). Immunohistochemical staining was performed manually using the standard avidin-biotin peroxidase complex technique with DAKO (LSAB kit, DAKO, Denmark). The primary antibody for ezrin (mouse mAbIgG1, clone 3C12, Neomarkers) was applied to the slides.
Histological and immunohistochemical evaluation was performed by one pathologist. Each stained slide was assessed and given a score, in which the intensity of the staining (no staining = 0, weak staining = 1, medium staining = 2, and strong staining = 3) and the percent of stained cells (0% = 0, 1%-24% = 1, 25%-49% = 2, 50%-74% = 3 and greater than 75% = 4) were multiplied. With the applied system, the maximum score was 12 (over 75% of the cells showing strong staining) and the minimum score was 0 (negative staining).
For detection of H pylori (H pylori), all the selected slides were Giemsa stained. Level of H pylori was assessed as 0-3 (no: 0, mild: 1, moderate: 2, severe: 3) according to the Sydney system.
SPSS for Windows version 11.0 was used for statistical analyses. Normality was checked for continuous variables. Data were expressed as mean ± SD (standard deviation), median minimum-maximum, n (number of cases) and percent (%). The Mann Whitney-U, t test and Spearman’s correlation tests were used where appropriate. Bonferroni's correction was applied (P < 0.05/n; where n = number of comparisons) when multiple comparisons were made. P value less than 0.05 or 0.02 was considered as significant in difference.
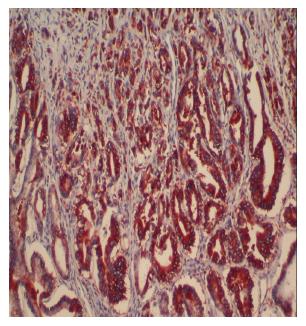
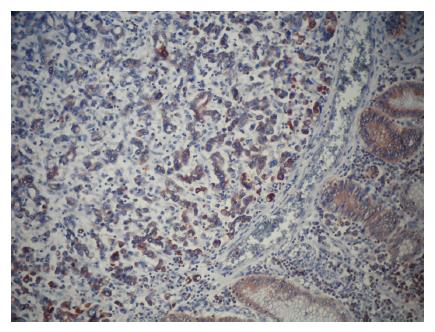
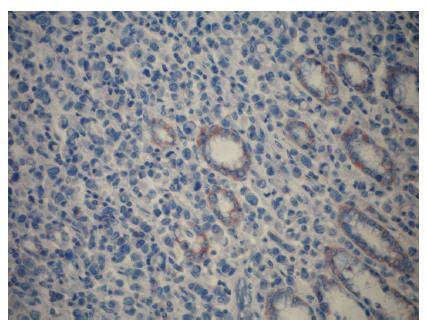
Positive staining for ezrin was observed in the epithelium of the gastric glands and tumor cells and in the cytoplasm of the cells. In noncancerous, normal gastric mucosa, glandular epithelium showed positive staining for ezrin. Ezrin immunostaining was positive in 43 cases (81.1%) of intestinal type and in 9 (40.9%) cases of diffuse type adenocarcinomas (P < 0.001) (Figures 1-3). Furthermore, the mean weighted ezrin score in the intestinal type adenocarcinoma was higher than that of the diffuse type (4.98 ± 3.841 vs 2.05 ± 3.00 respectively, P = 0.001).
As the gastric carcinomas were assessed as a whole group, there was no correlation between ezrin expression and TNM stage and histological grade in gastric carcinomas (P > 0.05) (Table 1). The expression of ezrin was positively correlated with the presence of H pylori and overall survival (P < 0.05) (Table 1). There was a negative correlation between ezrin and lymph node metastasis (Table 2), lymphovascular space invasion, and perineural invasion in all gastric carcinomas, but was not significant statistically (P > 0.05), while no association with depth of invasion, localization of tumor, and diameter of tumor, and distant metastasis (P > 0.05), (Table 2).
| İntestinal typeadenocarcinoma | Diffuse typeadenocarcinoma | Gastric adenocarcinoma(All groups) | İntestinal typeadenocarcinoma | Diffuse typeadenocarcinoma | ||||||
| Hp present | Hp absent | Hp present | Hp absent | Alive | Death | Grade 1 | Grade 2 | Grade 3 | Grade 3 | |
| Ezrin negative | 4 | 6 | 3 | 10 | 5 | 15 | 0 | 6 | 4 | 13 |
| Ezrin positive | 26 | 17 | 4 | 5 | 15 | 18 | 4 | 19 | 20 | 9 |
| P | < 0.05 | < 0.05 | > 0.05 | |||||||
| Intestinal type adenocarcinoma | Diffuse type adenocarcinoma | Intestinal type adenocarcinoma | Diffuse type adenocarcinoma | |||||
| With lymphnode metastasis | Without lymphnode metastasis | With lymphnode metastasis | Without lymphnode metastasis | With distantmetastasis | Without distantmetastasis | With distantmetastasis | Without distantmetastasis | |
| Ezrin negative | 9 | 1 | 12 | 1 | 1 | 8 | 1 | 12 |
| Ezrin positive | 31 | 12 | 8 | 1 | 11 | 31 | 2 | 7 |
| P | > 0.05 | > 0.05 | ||||||
In diffuse carcinoma (n = 22), ezrin expression was not associated with histological and clinical parameters while ezrin expression in intestinal type adenocarcinomas (n = 53) has a negative correlation (P < 0.05) with the size of tumor, but not with other parameters.
In this study, standard histological parameters such as grade, depth of tumor, metastatic lymph node status, lymphovascular space and perineural invasion, and diameter of tumor were significantly correlated with stage of tumor (P = 0.001, P = 0.007, P < 0.001, P < 0.001, P < 0.001, P < 0.001). The type of tumor showed no correlation with stage, depth of tumor, metastatic lymph node status, lymphovascular space and perineural invasion. The size of the tumor was significantly correlated with stage of tumor.
Sixty patients (80%) had lymph node metastasis and lymphovascular invasion and 15 patients (20%) had distant metastases such as liver and omentum. Fifty-three patients with gastric carcinoma were followed up for a mean (SD) duration of 12.37 mo. The median survival time was 18 mo in the group without ezrin expression, and 24 mo in the group with ezrin expression (P > 0.05). In diffuse and intestinal type carcinomas, the expression of ezrin was not correlated with the overall survival.
Despite identification of the new key regulatory molecules in metastasis and growth of tumor, metastasis is still an extremely complex and unclear process. To metastasize successfully into a clinically relevant mass, tumor cells must overcome a series of challenges. These include invasion into the surrounding tissue, extravasation into the lymphovascular space, arrive at a distant side, and intravasation into a new environment. In all of these stages, various regulatory molecules have to be expressed in a coordinated pattern. Ezrin is known to be a component of cell-surface structures that, together with ERM proteins, are involved in cell adhesion to the extracellular matrix, as well as in cell-cell interactions, receptor tyrosine-kinase signaling, signal transduction and interactions with the Akt-mediated cellular apoptotic mechanism. One of the functions of ezrin is to participate in the formation of cell-surface complexes, such as E-cadherin, integrin that mediates cell-cell and cell-extracellular matrix attachments[1-4].
Many molecular genetic studies in gastric carcinogenesis have shown that the development of intestinal and diffuse carcinoma follow two different pathways. For instance, microsatellite instability was found to be 64% in diffuse type, but only 17% in intestinal type carcinoma[9,10]. Loss of heterozygosity, mutation of APC gene and DCC gene are frequently observed in cancer of intestinal type , but seldom found in diffuse type. Similarly, the cadherin gene plays an important role in the carcinogenesis of diffuse type carcinoma which is characterized by invasion and high metastatic potential. In addition, the abnormal transcription of CD44 has found both types, although deletion of cadherin gene is 50% in intestinal type carcinoma[8].
Moilanen et al[12] reported that healthy ovarian epithelium showed strong ezrin immunreactivity, but weak or negative expression of ezrin in ovarian carcinoma was associated with shorter survival, histological grade, and advanced age of the patient. Controversially, in other studies, glial tumor, uveal malignant melanoma, and pancreatic carcinoma cells have shown high ezrin expression[5,6,11,13]. In human pancreatic adenocarcinoma cells, increased ezrin expression correlated with high metastatic potential. In this study, we observed strong ezrin immunoreactivity in normal, noncancerous gastric mucosa, but in the diffuse carcinoma group, the immunoreactivity of ezrin decreased as compared with normal mucosa. Ezrin expression showed inverse correlation with presence of metastatic lymph nodes, and lymphovascular space invasion in gastric carcinomas. Similar to our findings, the ezrin expression in human colon cancer was reported to decrease compared to normal tissues[14]. These seemingly contradictory results indicate that the expression of ezrin in various tumor types may be associated with different cell functions of ezrin. The previous studies implicated that the inactivation of ezrin caused a massive cell retraction, suggesting a constant exchange between the soluble and membrane-skeleton-associated ezrin, which drives cell spreading[15]. The suppression of ezrin led to the destruction of both cell-cell and cell-substrate adhesion whereas the overexpression of ezrin in insect cells enhanced cell adhesion[16,17]. Reduced cell-cell adhesion may be responsible for increased invasiveness and metastasis in malignant tumors. Considering the marked loss of adhesion of histologically diffuse carcinomas when compared to the intestinal carcinomas, the parallel loss of ezrin immunoreactivity may be meaningful perhaps causal in this regard.
Lim et al[18] demonstrated that H pylori infection increased the cell adhesion-related gene expression in gastric epithelial AGS cells and ezrin expression induced by H pylori infection. The expression of ezrin and possibly by other ERM proteins contributes to enhancement of cell-cell or cell-extracellular matrix adhesion of gastric epithelial cells. Lim et al[18] suggested that the differential expression of ezrin in H Pylori infection may play an important role in gastric carcinogenesis, including cell proliferation and cell adhesion. In the present study, we also found a positive correlation between H pylori status and expression of ezrin.
Generally, intestinal type gastric carcinomas have a better prognosis than their diffuse type counterparts. Environmental factors are believed to play a greater role in tumorigenesis in intestinal type carcinomas. H Pylori with its propensity resulting in atrophic gastritis is hypothesized to play a role in tumorigenesis of the intestinal type gastric cancer. However, there is a lack of information on the specific pathways and processes on H pylori-induced carcinogenesis. In this study, we have shown that H pylori infected gastric carcinomas have a greater expression of ezrin in the cells. If one takes into account of the ezrin’s role in numerous critical pathways of cellular adhesion and proliferation, this may be a genuine specific pathway in which H pylori may cause/initiate gastric carcinoma.
S- Editor Liu Y L- Editor Ma JY E- Editor Lu W
| 1. | Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 238] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Hunter KW. Ezrin, a key component in tumor metastasis. Trends Mol Med. 2004;10:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Yao X, Cheng L, Forte JG. Biochemical characterization of ezrin-actin interaction. J Biol Chem. 1996;271:7224-7229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Ohtani K, Sakamoto H, Rutherford T, Chen Z, Kikuchi A, Yamamoto T, Satoh K, Naftolin F. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002;179:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Akisawa N, Nishimori I, Iwamura T, Onishi S, Hollingsworth MA. High levels of ezrin expressed by human pancreatic adenocarcinoma cell lines with high metastatic potential. Biochem Biophys Res Commun. 1999;258:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27-39. [PubMed] |
| 8. | Chan AO, Luk JM, Hui WM, Lam SK. Molecular biology of gastric carcinoma: from laboratory to bedside. J Gastroenterol Hepatol. 1999;14:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Yasui W, Oue N, Kuniyasu H, Ito R, Tahara E, Yokozaki H. Molecular diagnosis of gastric cancer: present and future. Gastric Cancer. 2001;4:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Yasui W, Yokozaki H, Shimamoto F, Tahara H, Tahara E. Molecular-pathological diagnosis of gastrointestinal tissues and its contribution to cancer histopathology. Pathol Int. 1999;49:763-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chen Z, Fadiel A, Feng Y, Ohtani K, Rutherford T, Naftolin F. Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and ezrin translocation are stimulated by interleukin 1alpha and epidermal growth factor. Cancer. 2001;92:3068-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Moilanen J, Lassus H, Leminen A, Vaheri A, Bützow R, Carpén O. Ezrin immunoreactivity in relation to survival in serous ovarian carcinoma patients. Gynecol Oncol. 2003;90:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999;147:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112 Pt 18:3081-3090. [PubMed] |
| 15. | Lamb RF, Ozanne BW, Roy C, McGarry L, Stipp C, Mangeat P, Jay DG. Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr Biol. 1997;7:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003;46:165-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 283] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Lim JW, Kim H, Kim JM, Kim JS, Jung HC, Kim KH. Cellular stress-related protein expression in Helicobacter pylori-infected gastric epithelial AGS cells. Int J Biochem Cell Biol. 2004;36:1624-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |