Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 14, 2006; 12(6): 940-944
Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.940
Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.940
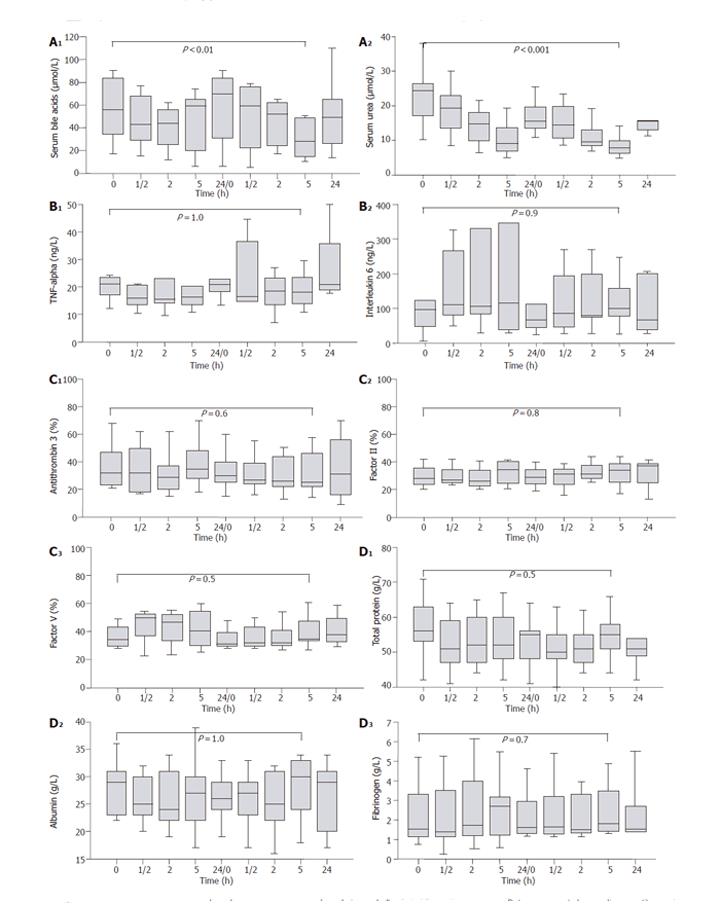
Figure 2 (A1-A2) Course of plasma bile acids and urea concentrations before, during and after 2 d of Prometheus® therapy.
Data are presented as median, quartiles, and minimum/maximum values. (B1-B2) Course of plasma TNF-α and IL-6 concentrations before, during and after 2 d of Prometheus® therapy. Data are presented as median, quartiles, and minimum/maximum values. (C1-C3) Course of antithrombin 3, factors II and V levels before, during and after 2 d of Prometheus® therapy. Data are presented as median, quartiles, and minimum/maximum values. (D1-D3) Course of plasma total protein, albumin, and fibrinogen before, during, and after 2 d of Prometheus® therapy. Data are presented as median, quartiles, and minimum/maximum values.
- Citation: Rifai K, Ernst T, Kretschmer U, Haller H, Manns MP, Fliser D. Removal selectivity of Prometheus: A new extracorporeal liver support device. World J Gastroenterol 2006; 12(6): 940-944
- URL: https://www.wjgnet.com/1007-9327/full/v12/i6/940.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i6.940