Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7699
Revised: November 12, 2006
Accepted: November 20, 2006
Published online: December 21, 2006
AIM: To explore the mechanisms of uncut Roux-en-Y gastrojejunostomy, which is used to decrease the occurrence of Roux stasis syndrome.
METHODS: The changes of myoelectric activity, mechanic motility and interstitial cells of Cajal (ICC) of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy were observed.
RESULTS: When compared with the cut group, the amplitude (1.15 ± 0.15 mV vs 0.48 ± 0.06 mV, P < 0.05) and frequency (14.4 ± 1.9 cpm vs 9.5 ± 1.1 cpm, P < 0.01) of slow waves and the incidence (98.2% ± 10.4% vs 56.6% ± 6.4%, P < 0.05) and amplitude (0.58 ± 0.08 mV vs 0.23 ± 0.06 mV, P < 0.01) of spike potential of the Roux limb in the uncut group were significantly higher. The migrating myoelectric complexes (MMC) phase III duration in the uncut group was significantly prolonged (6.5 ± 1.1 min vs 4.4 ± 0.8 min, P < 0.05), while the MMC cycle obviously shortened (42.5 ± 6.8 vs 55.3 ± 8.2 min, P < 0.05). Both gastric emptying rate (65.5% ± 7.9% vs 49.3% ± 6.8%, P < 0.01) and intestinal impelling ratio (53.4% ± 7.4% vs 32.2% ± 5.4%, P < 0.01) in the uncut group were significantly increased. The contractile force index of the isolated jejunal segment in the uncut group was significantly higher (36.8 ± 5.1 vs 15.3 ± 2.2, P < 0.01), and the expression of c-kit mRNA was significantly increased in the uncut group (0.82 ± 0.11 vs 0.35 ± 0.06, P < 0.01).
CONCLUSION: Uncut Roux-en-Y gastrojejunostomy may lessen the effects of operation on myoelectric activity such as slow waves, spike potential, and MMC, decrease the impairment of gastrointestinal motility, and remarkably increase the expression of c-kit mRNA.
- Citation: Zhang YM, Liu XL, Xue DB, Wei YW, Yun XG. Myoelectric activity and motility of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy. World J Gastroenterol 2006; 12(47): 7699-7704
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7699.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7699
Roux-en-Y anastomosis is a commonly used surgical procedure in gastroenterological surgery; however, more than one third of the patients who experience such an operation suffer Roux stasis syndrome[1,2]. It has been proposed that the occurrence of Roux stasis syndrome is related to the blockage of electro-conduction caused by cut of the jejunum[3]. Therefore, some researchers designed an uncut Roux-en-Y gastrojejunostomy[4], which was based on Billroth II subtotal gastrectomy, combined with jejunojejunostomy (Braun’s anastomosis) and occluded jejunal-gastric pathway. Theoretically, this surgical procedure can maintain the integrity of the intestinal canal and normal conduction of impulses, as well as make gastric contents to be drained directly into the intestinal tract along the movement direction of the alimentary tract. Due to blockade of gastric pathway, digestive fluids, such as bile, pancreatic juice, are unable to enter the stomach, and can merely be evacuated through the Braun’s anastomosis. Clinical practice has proved that this surgical procedure decreases the occurrence of Roux stasis syndrome[5,6]. In order to explore the underlying mechanisms, we observed the changes of myoelectric activity, mechanic motility and interstitial cells of Cajal (ICC) of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy.
Male Wistar rats (250 ± 20 g) were provided by the Animal Research Center of the First Clinical College of Harbin Medical University, Harbin, China. Fourteen rats were divided into two groups randomly. After the rats were anaesthetized with intraperitoneal injection of pentobarbital sodium (40 mg/kg body mass), resection of the distal stomach and reconstruction of the gastrointestinal tract were performed.
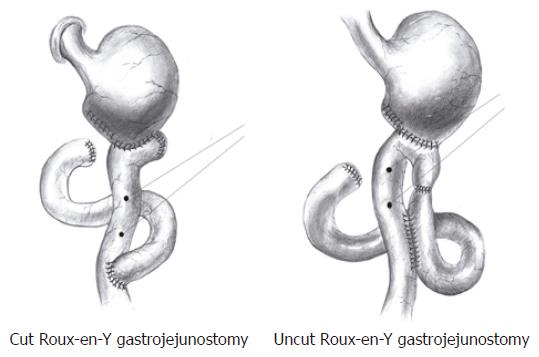
For the cut Roux-en-Y gastrojejunostomy, a standard Roux-en-Y gastrojejunostomy was undertaken (Figure 1). In brief, the jejunum was cut 1.0 cm distal to the ligament of Treitz, and the remaining gastric pouch was anastomosed to the aborad portion of the jejunum by an end-to-side procedure. The orad portion of the jejunum was anastomosed to the mid jejunum by an end-to-side procedure 2.0 cm distal to the gastrojejunostomy.
For the uncut Roux-en-Y gastrojejunostomy (Figure 1), an end-to-side gastrojejunostomy was constructed approximately 4.0 cm distal to the ligament of Treitz. The jejunal lumen was occluded 0.5 cm proximal to this anastomosis, and a side-to-side Braun’s jejunojejunostomy was made approximately 2.0 cm distal to this anastomosis.
One month after the operation, electric activity of the Roux limb was studied. Then the rats were sacrificed and gastrointestinal motility and levels of c-kit mRNA of the Roux limb were detected.
As shown in Figure 1, a couple of monopolar silver-silver chloride electrodes were implanted on the seromuscular layer of the Roux limb, which was spaced evenly 1.0 cm apart. The electrodes were connected by insulated leads to a multipinned socket contained in a plastic cannula, the outer end of which was exteriorizd and the inner end anchored to the left lower quadrant of the abdominal wall.
One month after the operation, electric activity was recorded during fasting conditions. The electrodes were connected to a 3802 biological signal amplifier system and the amplified analogue signals were converted to digital signals using NSA4 data acquisition system, sampled at 100 Hz, and displayed in real time on a VGA monitor while simultaneously stored on magnetic media for later analysis using Pclab software (Version 2.2.0).
The amplitude and frequency of slow waves, as well as incidence and amplitude of spike potential of jejunal smooth muscle were recorded. Amplitude of spike potential was represented by the maximum value of spike potential cluster.
Incidence of spike potential (%) = (number of spike potential cluster/number of slow wave) × 100%.
Each rat took orally 0.3 mL of the mixture of Arabian gel and active charcoal powders. Thirty minutes later, the rats were sacrificed and a laparotomy was performed. Then the stomach from proventriculus to the gastrointestinal stoma was cut and its gross and net mass were weighed. In addition, intact small intestines (from gastrointestinal stoma to ileocecal junction) were took out, and placed on paper without pull strength. Then the lengths from gastrointestinal stoma to the proximal end of charcoal paste reach and to ileocecal junction were measured.
Gastric emptying rate (%) = [1- (gastric gross mass-gastric net mass)/mass of charcoal paste] × 100%
Small intestinal impelling ratio (%) = (distance of charcoal paste reach/length of total intestine) × 100%
About 2.0 cm Roux limb was took off and put into 10 mL Tyrode’s solution, which had been pre-saturated by a mixed gas (95% O2 + 5% CO2). One end of the jejunal segment was tied on a specimen fixation hook in the water-bath, and the other end was connected with a tonotransducer, then the tonotransducer was linked with the CH1 input interface of BL-New Century bio-signal collecting and processing system. The motility changes of the jejunal segment were recorded. During the experiment, the temperature of Tyrode’s solution should be kept at 37.0°C ± 0.5°C, and continuously ventilated at a constant speed. Contractile force index = contraction amplitude × contraction frequency.
Total RNA was isolated from one part of the Roux limb using Trizol reagent (Invitrogen, USA) and was reversely transcribed into cDNA according to the instruction of the kit (Promega, USA). The resulting cDNA was used as a template for subsequent polymerase chain reaction (PCR). Samples were then heated to 94°C for 5 min and cycled 40 times at 94°C for 15 s, 48°C for 60 s, and 72°C for 90 s, and finally extended at 72°C for 10 min. The primer set designed for PCR amplification of rat c-kit mRNA was 5’-AGCAAGAGTTAACGATTCCGGAG-3’ and 5’-CCAGAAAGGTGTAAGTGCCTCCT-3’, and the predicted size of the PCR product was 344 bp according to the reported sequence of rat c-kit mRNA. The primer of housekeeping gene β-actin (348 bp) was 5’CATCACCATTGGCAATGAGCG 3’ and 5’CTAGAAGCATTTGCGGTCGGAC 3’. The PCR products were measured using Quantity One software.
Data were expressed as mean ± SD. Comparison between groups was performed with analysis of variance (ANOVA) and Student-Newman-Keuls test (q test). Statistical significance was assumed at P < 0.05.
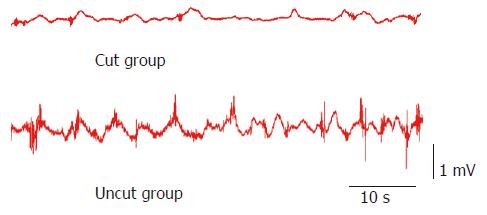
Slow waves were found in both the uncut group and cut group as shown in Figure 2, which were composed of an initial three phase complex potential and a secondary isopotentiality. When compared with the cut group, the amplitude (1.15 ± 0.15 mV vs 0.48 ± 0.06 mV, P < 0.05) and frequency (14.4 ± 1.9 cpm vs 9.5 ± 1.1 cpm, P < 0.01) of slow waves were significantly higher in the uncut group. Spike potential was overlapped with slow wave potential and emerged following the three phase complex potential. The incidence (98.2 ± 10.4% vs 56.6 ± 6.4%, P < 0.05) and amplitude (0.58 ± 0.08 mV vs 0.23 ± 0.06 mV, P < 0.01) of spike potential in the uncut group were significantly higher than the cut group (Table 1).
| Group (n = 7) | Frequency of | Amplitude of | Incidence of | Amplitude of | MMC phase III | MMC |
| slow wave | slow wave | spike potential | spike potential | duration | cycle | |
| (cpm) | (mV) | (%) | (mV) | (min) | (min) | |
| Cut group | 9.5 ± 1.1 | 0.48 ± 0.06 | 56.6 ± 6.4 | 0.23 ± 0.06 | 4.4 ± 0.8 | 55.3 ± 8.2 |
| Uncut group | 14.4 ± 1.9a | 1.15 ± 0.15b | 98.2 ± 10.4a | 0.58 ± 0.08b | 6.5 ± 1.1a | 42.5 ± 6.8a |
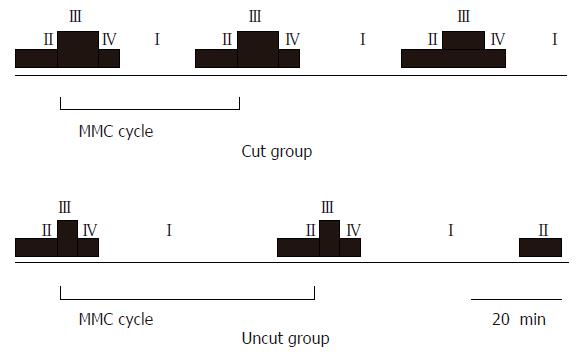
Slow waves were occasionally loaded with spike potential. According to the spike potential loading situation, the periodicity of electric activity could be observed, namely, MMC. At the phase III of MMC, large-amplitude and clustered spike potentials almost covered all slow waves, in which the lasting time was defined as MMC phase III duration and the interval time between two MMC phase III was defined as an MMC cycle. MMC phase III duration in the uncut group was significantly prolonged when compared with the cut group (6.5 ± 1.1 min vs 4.4 ± 0.8 min, P < 0.05), while the MMC cycle obviously shortened (42.5 ± 6.8 vs 55.3 ± 8.2 min, P < 0.05) (Figure 3 and Table 1).
Gastric emptying and food-transmitting rate of rats were reflected by gastric emptying rate and intestinal impelling ratio, respectively. A lower relative charcoal residual rate represents a higher gastric emptying rate, which means a better gastric emptying capacity. On the other hand, the higher the intestinal impelling ratio, the faster the intestinal transmission rate. When compared with the cut group, both gastric emptying rate (65.5 ± 7.9% vs 49.3 ± 6.8%, P < 0.01) and intestinal impelling ratio (53.4 ± 7.4% vs 32.2 ± 5.4%, P < 0.01) in the uncut group were significantly increased, suggesting that gastrointestinal motility was impaired more seriously in the cut group (Table 2).
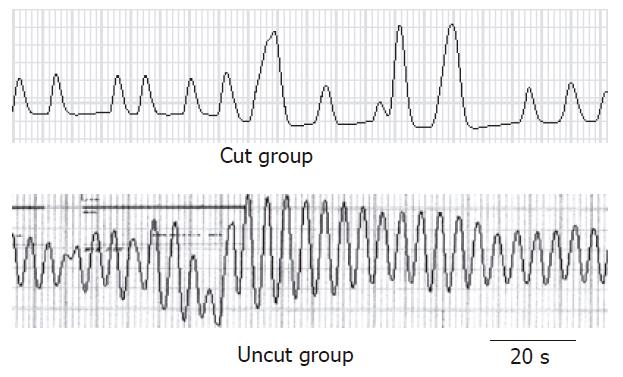
Spontaneous contraction could be observed in isolated jejunal segment as shown in Figure 4. The contractile force index in the uncut group was significantly higher than that in the cut group (36.8 ± 5.1 vs 15.3 ± 2.2, P < 0.01) (Table 2).
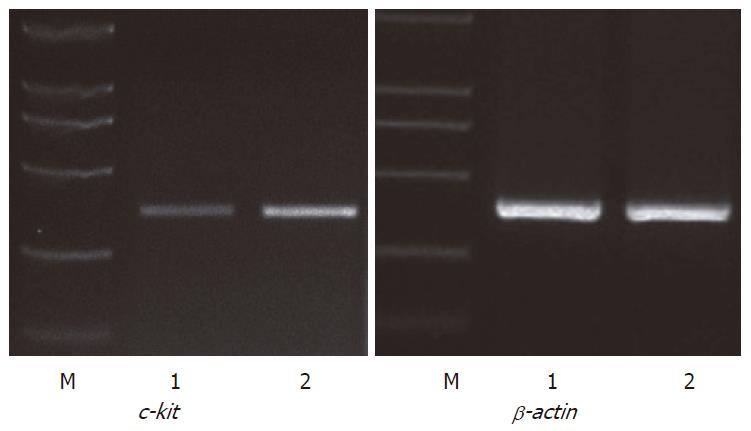
To evaluate the quantity and function of ICC, c-kit mRNA expression was examined using semi-quantitive RT-PCR. The 348 bp band of β-actin confirmed the integrity of the cDNA obtained from each sample. For c-kit a 344 bp band was detectable at variable levels (Figure 5). The expression of c-kit mRNA was significantly increased in the uncut group compared with that of the cut group (0.82 ± 0.11 vs 0.35 ± 0.06, P < 0.01) (Table 2).
Patients undergoing Roux-en-Y anastomosis complain of upper abdominal fullness and distension, abdominal pain, nausea or vomiting, and these symptoms are aggravated after meals. Such a syndrome is known as Roux Stasis Syndrome. Van der[8] reported that 26 of 37 (70%) patients experienced such a syndrome. The high incidence has attracted much attention in the research circle. Schippers’ findings showed that intestinal movement of patients with Roux stasis syndrome is disordered and lacks regular MMC in interdigestive phase, suggesting that the small intestine may lose the stimulation of pacemaker potential initiated from the duodenum due to destruction of the continuity of the small intestine[9]. Thus, it may be helpful to relieve Roux stasis syndrome by keeping the integrity of the nerves and muscles between duodenal pacemaker potential and the Roux limb. First, back flow of the bile and pancreatic juice through ascending Roux limb should be prevented; second, the integrity of muscular layer of Roux limb should be maintained. Uncut Roux-en-Y gastrojejunostomy could meet these requirements. Chen[10] showed that the reconstitution of the gastrointestinal tract with Roux-en-Y gastrojejunostomy is more effective than with Billroth II operation on gastric emptying. Vogel[11] carried out a re-operation to reestablish the continuity of the small intestine for patients with Roux stasis syndrome. As a result, these patients could take food normally post operation without biliary vomiting or burning epigastric pain. In order to explore the underlying mechanisms, we carried out a comparative study between uncut Roux-en-Y gastrojejunostomy and traditional cut Roux-en-Y gastrojejunostomy, and observed the changes of myoelectric activity and motility.
Slow waves, also known as basic or pacemaker potential, are the basis of various electric activities of the gastrointestinal tract, of which the major function is to enhance the excitability of smooth muscles. Cells in each part of the gastrointestinal tract have spontaneous electric activity, and the slow waves attenuate along the gastrointestinal tract; the slow wave of the orad portion with a higher frequency has the inherent effect to drive the lower frequency of the aborad portion, which makes the rhythm of the aborad portion to keep pace with that of the orad portion. After Roux-en-Y gastrojejunostomy, the jejunum is cut off, which causes the frequency of slow waves diminished due to intervention of normal transmission of slow waves. This viewpoint was proved by the current study. Because the intestinal canal was severed in the cut group, the frequency of slow waves was lower compared with the uncut group (P < 0.05). It indicated that the excitability of smooth muscles in the uncut group was higher than that in the cut group.
Spike or active potentials are able to trigger contractions of smooth muscles, and the contraction intensity of intestinal smooth muscles has a positive relationship with the incidence of spike potential. This study showed that the incidence of spike potential in the uncut group was higher than that in the cut group (P < 0.01). It suggests that the application of uncut Roux-en-Y gastrojejunostomy can decrease the influence on the incidence of spike potential and impairment of the contraction activity of the intestine.
Occurred periodically, MMC is clustered, large-amplitude spike potential activity, which can migrate from the duodenum to terminal ileum. MMC consists of four phases: phase I is characterized by slow waves without spike potential; phase II and IV are characterized by a small quantity of spike potential loaded on some slow waves; phase III shows large-amplitude, clustered spike potential loaded on all slow waves. Phase III electric activity corresponds to a series of propagated contractions, which produce intensive impelling action for small intestinal contents, and periodically scavenge gastrointestinal contents between meals. Deficiency or shortened MMC phase III would lead to accumulation of remnant digestive products, cast-off cell, and secretory juice, and facilitate the propagation of bacteria in the small intestine[12]. Le Blanc-Louvry[13] reported that traditional Roux-en-Y operation may shorten MMC phase III duration. In the current study, the duration of MMC phase III significantly prolonged in the uncut group (P < 0.01). Previous findings have shown that uncut Roux-en-Y surgical procedure can relieve Roux stasis syndrome by increasing the frequency of MMC occurrence, prolonging the duration of MMC phase III, and enhancing the clearance of interdigestive remnants. Some studies on gastrointestinal dynamics such as gastric emptying and small intestine impelling ratio also showed that the gastrointestinal motility of experimental animals was significantly improved in the uncut Roux-en-Y group, which is consistent with the studies of Ward[14].
When eliminating the influence of organism adjustment factors such as nerves, and body fluids, we found that there was still some difference in intestinal motility between the two groups. To explore this inner mechanism, we detected the ICC changes of different operations with RT-PCR technique.
ICC is a kind of special interstitial cell in gastrointestinal tract, which has a close relationship with the nervous system. The cell can produce slow waves itself, and thus it has been thought as the pacemaker cell of the gastrointestinal tract[15,16]. It has been shown that ICC plays an important role in the gastrointestinal electrophysiology, dynamo-development and dyskinesis[17,18]. In addition, abnormal expression of ICC has been discovered in some gastrointestinal tract diseases such as gastroparesis[19], chronic idiopathic intestinal pseudo-obstruction[20], slow transit constipation, ulcerative colitis[21] and Crohn’s disease[22]. Won[23] observed that the expression of ICC decreased in the animal model of bowel obstruction. c-kit is an important marker to identify ICC. It is a kind of transmembrane protein with tyrosine kinase function. During embryonic development period, c-kit signal can make ICC precursor develop to myenteric ICC, and finally to be the pacemaker cell of the intestinal tract; whereas those non-connected with c-kit signal develop to longitudinal muscle layer[24]. With respect to the long-term maintenance of ICC function, c-kit signal is of great importance. Chronic loss or deficiency of this signal transduction may cause blockage or deprivation as well as functional impairment of ICC network[25,26]. Animals with c-kit mutant defect have difficulties to produce complete, regular spontaneous slow waves by gastrointestinal tissue[27]. Therefore, c-kit reflects the quantity and function of ICC[28]. In this study, c-kit mRNA in the uncut group was significantly higher than that in the cut group, which may result from decreased stimulation of the high-frequency electric activity of the orad portion in the cut group.
In summary, compared with traditional cut Roux-en-Y gastrojejunostomy, uncut Roux-en-Y gastrojejunostomy may lessen the effects of operation on myoelectric activity such as slow waves, spike potential, and MMC, decrease the impairment of gastrointestinal motility, and remarkably improve the ICC quantity and function.
Roux-en-Y anastomosis is a commonly used surgical procedure in gastroenterological surgery; however, more than one third of the patients who experience such an operation suffer Roux stasis syndrome. Therefore, it is important to develop a moderate surgical procedure to control these kinds of complications.
The etiology and treatment of Roux stasis syndrome are yet to be clarified.
We studied the myoelectric activity, gastrointestinal motility, and c-kit, which represent the ICC quantity and function.
Uncut Roux-en-Y gastrojejunostomy has already been used in clinical practice, and this article will provide more rationale for its application.
Uncut Roux-en-Y gastrojejunostomy is based on Billroth II subtotal gastrectomy, combined with Braun’s jejunojejunostomy and occluded jejunal-gastric pathway. Theoretically, this surgical procedure can maintain the integrity of intestinal canal and normal conduction of impulse, as well as make gastric contents to be drained directly into the intestinal tract along the movement direction of the alimentary tract.
The authors performed an experimental study in rats on myoelectric activity and motility of the Roux limb following cut versus uncut Roux-en-Y gastrojejunostomy.
The research background of the study is clearly defined. The presentation of the results and their discussion are adequate. Results provide sufficient evidence for the conclusions drawn. Overall, the content of the manuscript is interesting and could be valuable for a broad readership.
S- Editor Pan BR L- Editor Zhu LH E- Editor Bi L
| 1. | Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am. 1992;72:445-465. [PubMed] [Cited in This Article: ] |
| 2. | Qin XY, Lei Y, Liu FL. Effects of two methods of reconstruction of digestive tract after total gastrectomy on gastrointestinal motility in rats. World J Gastroenterol. 2003;9:1051-1053. [PubMed] [Cited in This Article: ] |
| 3. | Zonca S, Rizzo P. Alteration of the Roux Stasis syndrome by an isolated Roux limb: correlation of slow waves and clinical course. Am Surg. 1999;65:666-672. [PubMed] [Cited in This Article: ] |
| 4. | Kummer EW, Gerritsen JJ, Brummelkamp WH. The cut-closed-reconnected Roux loop. Am J Surg. 2000;179:141-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Tu BL, Kelly KA. Surgical treatment of Roux stasis syndrome. J Gastrointest Surg. 1999;3:613-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mon RA, Cullen JJ. Standard Roux-en-Y gastrojejunostomy vs. "uncut" Roux-en-Y gastrojejunostomy: a matched cohort study. J Gastrointest Surg. 2000;4:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Gilani AH, Ghayur MN. Pharmacological basis for the gut stimulatory activity of Raphanus sativus leaves. J Ethnopharmacol. 2004;95:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Van der Mijle HC, Kleibeuker JH, Limburg AJ, Beekhuis H, Lamers CB, van Schilfgaarde R. Role of vagal dysfunction in motility and transit disorders of jejunal Roux limb after Roux-en-Y gastrojejunostomy. Dig Dis Sci. 1994;39:827-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Schippers E, Willis S, Ruckdeschel G, Schumpelick V. Small intestinal myoelectrical activity and bacterial flora after Roux-en-Y reconstruction. Br J Surg. 1996;83:1271-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 193] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Vogel SB, Drane WE, Woodward ER. Clinical and radionuclide evaluation of bile diversion by Braun enteroenterostomy: prevention and treatment of alkaline reflux gastritis. An alternative to Roux-en-Y diversion. Ann Surg. 1994;219:458-465; discussion 465-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Le Blanc-Louvry I, Ducrotté P, Lemeland JF, Metayer J, Denis P, Ténière P. Motility in the Roux-Y limb after distal gastrectomy: relation to the length of the limb and the afferent duodenojejunal segment--an experimental study. Neurogastroenterol Motil. 1999;11:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Le Blanc-Louvry I, Ducrotté P, Peillon C, Michel P, Chiron A, Denis P. Roux-en-Y limb motility after total or distal gastrectomy in symptomatic and asymptomatic patients. J Am Coll Surg. 2000;190:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Klaus A, Hinder RA, Nguyen JH, Nelson KL. Small bowel transit and gastric emptying after biliodigestive anastomosis using the uncut jejunal loop. Am J Surg. 2003;186:747-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Albertí E, Mikkelsen HB, Larsen JO, Jiménez M. Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid- and distal-colon of the rat. Neurogastroenterol Motil. 2005;17:133-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yoneda S, Fukui H, Takaki M. Pacemaker activity from submucosal interstitial cells of Cajal drives high-frequency and low-amplitude circular muscle contractions in the mouse proximal colon. Neurogastroenterol Motil. 2004;16:621-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Li CX, Liu BH, Tong WD, Zhang LY, Jiang YP. Dissociation, culture and morphologic changes of interstitial cells of Cajal in vitro. World J Gastroenterol. 2005;11:2838-2840. [PubMed] [Cited in This Article: ] |
| 18. | Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J Gastroenterol. 2004;10:1227-1230. [PubMed] [Cited in This Article: ] |
| 19. | Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Tong WD, Liu BH, Zhang LY, Xiong RP, Liu P, Zhang SB. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2005;20:363-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Porcher C, Baldo M, Henry M, Orsoni P, Julé Y, Ward SM. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn's disease. Am J Gastroenterol. 2002;97:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 695] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 25. | Kitamura Y, Hirotab S. Kit as a human oncogenic tyrosine kinase. Cell Mol Life Sci. 2004;61:2924-2931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Daniel EE, Willis A, Cho WJ, Boddy G. Comparisons of neural and pacing activities in intestinal segments from W/W++ and W/W(V) mice. Neurogastroenterol Motil. 2005;17:355-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602-G611. [PubMed] [Cited in This Article: ] |
| 28. | Ordög T, Redelman D, Miller LJ, Horváth VJ, Zhong Q, Almeida-Porada G, Zanjani ED, Horowitz B, Sanders KM. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am J Physiol Cell Physiol. 2004;286:C448-C456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |