Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2006; 12(46): 7497-7502
Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7497
Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7497
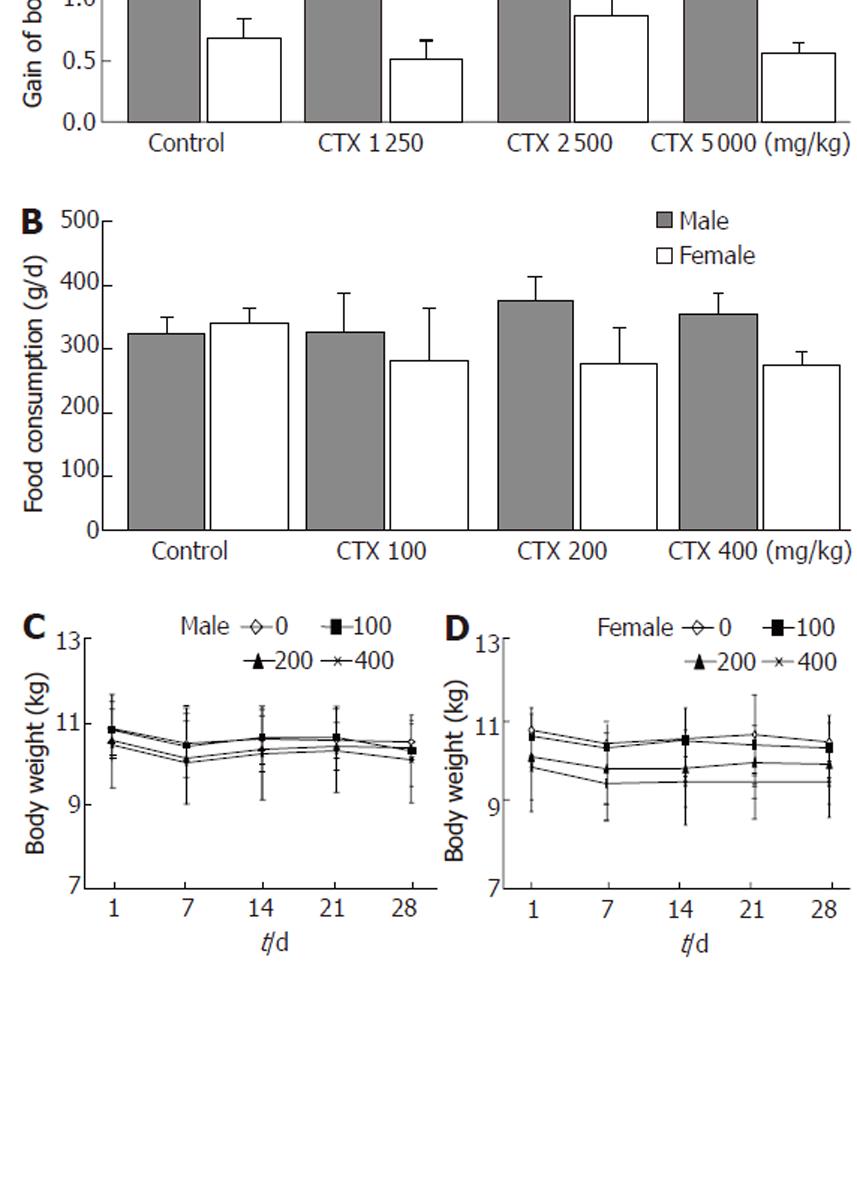
Figure 2 Body weight and food consumption.
A: Final gains in body weight for each group administered with indicated Chunggan extract (CGX) after 2 wk of the acute toxicity experiment (n = 2 males and 2 females); B: Average daily food consumption for each group (n = 3 males and 3 females) during the subacute toxicity experiment; C: Change in body weight during 4 wk of the subacute toxicological experiment, shown separately for males (n = 3) and females (n = 3). Values represent the mean ± SD.
- Citation: Choi WJ, Shin JW, Son JY, Seo DS, Park HS, Han SH, Sung HJ, Cho JH, Cho CK, Yoo HS, Lee YW, Son CG. Toxicological study of the hepatotherapeutic herbal formula, Chunggan extract, in beagle dogs. World J Gastroenterol 2006; 12(46): 7497-7502
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7497