Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7361
Revised: August 28, 2006
Accepted: November 1, 2006
Published online: December 7, 2006
AIM: To study the molecular forms of trefoil factor 1 (TFF1) in normal gastric mucosa and its expression in normal and abnormal gastric tissues (gastric carcinoma, atypical hyperplasia and intestinalized gastric mucosa) and the role of TFF1 in the carcinogenesis and progression of gastric carcinoma and its molecular biological mechanism underlying gastric mucosa protection.
METHODS: The molecular forms of TFF1 in normal gastric mucosa were observed by Western blot. The expression of TFF1 in normal and abnormal gastric tissues (gastric carcinoma, atypical hyperplasia and intestinalized gastric mucosa) was also assayed by immunohistochemical method. The average positive AO was estimated by Motic Images Advanced 3.0 software.
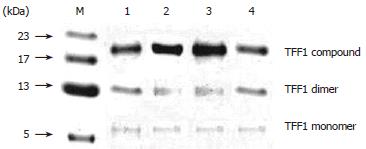
RESULTS: Three patterns of TFF1 were found in normal gastric mucosa: monomer, dimmer, and TFF1 compound whose molecular weight is about 21 kDa. The concentration of TFF1 compound was the highest among these three patterns. TFF1 was expressed mainly in epithelial cytoplasm of the mucosa in gastric body and antrum, especially around the nuclei. The closer the TFF1 to the lumen, the higher the expression of TFF1. The expression of TFF1 in peripheral tissue of gastric carcinoma (0.51 ± 0.07) was higher than that in normal gastric mucosa (0.44 ± 0.06, P < 0.001). The expression of TFF1 in gastric adenocarcinoma was positively related to the differentiation of adenocarcinoma. The lower the differentiation of adenocarcinoma was, the weaker the expression of TFF1. No TFF1 was expressed in poorly-differentiated adenocarcinoma. The expression of TFF1 in moderately-well differentiated adenocarcinoma (0.45 ± 0.07) was a little lower than that in normal mucosa (P > 0.05). The expression of TFF1 in gastric mucosa with atypical hyperplasia (0.57 ± 0.03) was significantly higher than that in normal gastric mucosa (P < 0.001). No TFF1 was expressed in intestinalized gastric mucosa. There was no statistically significant difference between the expressions of TFF1 in gastric mucosa around the intestinalized tissue (0.45 ± 0.07) and normal gastric mucosa (P > 0.05).
CONCLUSION: TFF1 is expressed mainly in epithelial cytoplasm of the mucosa in gastric body and antrum. Its main pattern is TFF1 compound, which may have a greater biological activity than monomer and dimer. The expression of TFF1 in peripheral mucosa of gastric ulcer is higher than that in mucosa 5 cm beyond the ulcer, indicating that TFF1 plays an important part in protection and restitution of gastric mucosa. The expression of TFF1 is increased in peripheral tissues of gastric carcinoma and gastric mucosa with atypical hyperplasia, but is decreased in cancer tissues, implying that TFF1 may be related to suppression and differentiation of carcinoma. The weaker expression of TFF1 in poorly-differentiated carcinoma may be related to the destruction of glands and cells in cancer tissues and the decrease in secretion of TFF1.
- Citation: Ren JL, Luo JY, Lu YP, Wang L, Shi HX. Molecular forms of trefoil factor 1 in normal gastric mucosa and its expression in normal and abnormal gastric tissues. World J Gastroenterol 2006; 12(45): 7361-7364
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7361
Trefoil factor 1 (TFF1) is a member of the trefoil factor family, which is a group of small molecule polypeptides mainly secreted by gastrointestinal mucous cells. TFF1 is mainly expressed in epithelial cytoplasm of the mucosa in gastric body and antrum[1]. The specificity of expression disappears in case of gastrointestinal mucosa injury[2]. TFF1 can be expressed in the whole injured mucosa,and its expression in injured mucosa is much higher than that in normal mucosa[3]. The purpose of this experiment was to observe the expression and patterns of TFF1 in normal and abnormal gastric tissues (gastric carcinoma, atypical hyperplasia, and intestinalization) and to investigate the role TFF1 in the carcinogenesis and progression of gastric carcinoma and its molecular biological mechanism underlying gastric mucosa protection.
Gastric antral specimens were obtained from a cohort undergone upper gastrointestinal endoscopy without abnormal appearance at Zhongshan Hospital affiliated to Xiamen University in June 2003. Two specimens were taken from each person and preserved in liquified nitrogen.
Paraffin specimens were obtained from 160 patients who underwent gastroscopic biopsy or radical gastric carcinomectomy during January 2002-June 2003 in Zhongshan Hospital affiliated to Xiamen University. The age of the patients ranged from 40 to 70 (53.2 ± 6.5) years. Of these 160 patients, 35 had normal gastric antrum mucosa, esophageal, gastric and duodenal tissues in gastroscopy and normal gastric mucosa in pathologic examination; 38 were diagnosed as gastric adenocarcinoma by pathologic examination; 36 as atypical hyperplasia; 35 as intestinalized gastric mucosa; and 20 as esophageal squamous carcinoma.
Sheep anti-human TFF1 multiclonal antibody (first antibody) and second antibody were purchased from Beijing Zhongshan Jinqiao Biotechnology Company, Ltd. EDTA, SDS and β-mercaptoethanol were obtained from Sigma Company. Acrylamide, protein-determination reagent and NC film were bought from Bio-Rad Company. ECL Western blotting reagents were from Amersham Company. Mice anti-human TFF1 monoclonal antibody, S-P super sensitive kit and DAB display kit were from Fuzhou Maixin Biotechnology Development Company.
Normal gastric mucosa specimens were added into buffer solution (containing 0.2 mmol/L sucrose, 10 mmol/L Tris, 1.5 mmol/L EDTA, 20 mmol/L β-mercaptoethanol, 1% PMSF, 1% DTT) preset at 4°C. After the solution was uniformly mixed, protein was extracted and its concentration was measured. One hundred and twenty ug of protein from each specimen was added into buffer solutions of the same volume. The solutions were boiled at 100°C for 5 min. The proteins were isolated by acrylamide gel electrophoresis (5% aggregation gel of 70 V and 20% isolation gel of 120 V) for about 5 h. NC films were metastasized by 250 mA electricity in icy water for 90 min by wet metastasis method. The proteins were kept in confining liquid at room temperature for 30 min, inoculated in 1:200 first antibody at room temperature for 3 h and cleansed by PBS-T, and then inoculated in 1:4000 second antibody for 3 h, cleansed by PBS-T, and exposed to ECL in dark room.
Specimens from normal and abnormal gastric tissues (gastric carcinoma, atypical hyperplasia, and intestinalized gastric mucosa) were fixed in 10% formaldehyde, routinely dehydrated, cleaned, infiltrated with wax, embedded and made into serial 4 μm thick sections. The sections were dewaxed, stained with SP method, displayed by DAB, stained again with hematoxylin and blown dry. The specific operations were carried out following the instructions on the S-P test kit.
All the data were analyzed by t-test using SPSS 10.0 software, and expressed as mean ± SD.
Three patterns of TFF1 were found in normal gastric mucosa: monomer, dimer and TFF1 compound whose molecule weight is about 21 kDa. Among these three patterns, the concentration of TFF1 compound was the highest, followed by that of dimer, and monomer (Figure 1).
Each section was photographed under 100 × high-power microscope. Motic Imaged Advanced 3.0 software was used to estimate the average positive AO of 20 glands randomly selected to reflect the intensity of TFF1 expression. The higher the AO was, the stronger the expression was.
TFF1 was expressed mainly in epithelial cytoplasm of the mucosa in gastric body and antrum, especially around the nuclei. The cytoplasm of positive cells was buffy after staining. The closer the TFF1 to the lumen, the deeper the color was. The expression of TFF1 in peripheral tissues of gastric carcinoma (0.51 ± 0.07) was higher than that in normal gastric mucosa (0.44 ± 0.06, P < 0.001). The expression of TFF1 in gastric adenocarcinoma was positively related to the differentiation of adenocarcinoma. The lower the differentiation of adenocarcinoma was, the weaker the expression of TFF1 was. No TFF1 was expressed in poorly-differentiated adenocarcinoma. The expression of TFF1 in moderately-well differentiated adenocarcinoma (0.45 ± 0.07) was a little lower than that in normal mucosa (P > 0.05). The expression of TFF1 in gastric mucosa with atypical hyperplasia (0.57 ± 0.03) was significantly higher than that in normal gastric mucosa (P < 0.001). No TFF1 was expressed in intestinalized gastric mucosa. There was no statistically significant difference between the expressions of TFF1 in gastric mucosa around the intestinalized tissues (0.45 ± 0.07) and normal gastric mucosa (P > 0.05). No TFF1 was expressed in 20 specimens from patients with esophageal carcinoma (including peripheral tissues of carcinoma) (Table 1).
| Classification | n | Averagepositive A |
| Normal | 35 | 0.44 ± 0.06 |
| Low-differentiated gastric carcinoma | 27 | None |
| Middle and highly differentiated gastric carcinoma | 11 | 0.41 ± 0.07a |
| Peripheral tissues of gastric carcinoma | 38 | 0.51 ± 0.07b |
| Atypical hyperplasia | 36 | 0.57 ± 0.03b |
| Intestinalization | 35 | None |
| Peripheral tissues of intestinalized mucosa | 35 | 0.45 ± 0.07a |
| Esophageal carcinoma | 20 | None |
| Peripheral tissues of esophageal carcinoma | 20 | None |
Trefoil factor family (TFF) is a group of small molecule polypeptides and mainly secreted by gastrointestinal mucous cells. At present there are three kinds of trefoil peptides found in mammals, which are breast cancer-associated peptide (pS2 or TFF1), spasmolytic polypeptide (SP or TFF2) and intestinal trefoil factor (ITF or TFF3). These three patterns of TFF have a special structure, P-structure domain. This structure is composed of 38-39 amino acids sequenced by 6 highly conservative cysteine residues linked by three intramolecular disulfide bonds which make the whole peptide chain twisted and folded into trefoil-shaped structure[1]. Studies at home and abroad have proven that TFF plays an important role in protection and restitution of gastrointestinal mucosa[4-6].
TFF1, a member of TFF, was obtained from MCF-7 cell line of human mammary carcinoma by estrogen induction[7]. Each TFF1 molecule is composed of 60 amino acids and its molecular weight is 6674 kDa, including[8] cysteine residues, of which 6 take part in the constitution of P-structure domain and the 7th lies in the third base to the end of carboxyl side, i.e. Cys58. Chadwick et al[5] replaced Cys58 of recombinant TFF1 protein with Ser58 and analyzed TFF1 containing Ser58 by equilibrium ultracentrifugation, gel filtration, polyacrylamide gel electrophoresis, and mass spectrum. They found that homologous dimer could present in TFF1, but not in TFF1 analog containing Ser58, suggesting that Cys58 can form intermolecular disulfide bond and shape the dimer. In the present study, three patterns of TFF1 were found in normal gastric mucosa: monomer (6.5 kDa), dimmer and TFF1 compound whose molecule weight is about 21 kDa. The concentration of TFF1 compound was the highest among these three patterns, followed by that of dimer and monomer, indicating that the biological activity of TFF1 may be related to the formation of homologous dimmer or other oligomers composed of heterogenous proteins.
At present, there are two hypotheses concerning the mechanism underlying trefoil peptide protection in gastric mucosa. Trefoil peptide could bind to glycoprotein in mucus to form stable gel compounds, which could reinforce the mucous gel layer and decrease injury to mucosa by harmful substance in gastric surface and mechanical stress, etc. In vivo and vitro studies have shown that trefoil peptide can increase viscosity of mucous gel layer, decrease the ability of H+ to penetrate mucous gel layer and injuries to gastric mucosa[9]. Trefoil peptide is likely to accomplish its biologic function by binding to its receptor or transport protein. To find the possible receptor or transport protein and explore their mechanism are one of the hot topics in international studies on trefoil peptide[10-12]. TFF1 compound found in this study is likely composed of TFF1 and its receptor, transport protein or some glycoproteins. However, different molecules binding to TFF1 have been reported by different scholars. This diversity might be related to different experimental conditions, the ability of trefoil peptide to bind to different molecules, and the unstable structure of molecules binding to trefoil peptide. All the molecules are parts of the possible binding molecule. No consensus has been reached upon the real causes of the diversity. Moreover, no breakthrough has been made in finding the possible receptor or transport protein.
TFF1 is mainly expressed in epithelial cells of the mucosa in gastric body and antrum, but the specificity of expression disappears in pathological tissues[2]. TFF1 can be expressed in injured parts of gastrointestinal mucosa[3], much higher than that in normal mucosa. In the present study, the expression of TFF1 was higher in mucosa with peptic ulcer or gastritis, mucosa around gastric carcinoma and mucosa with stress ulcer induced by aspirin in rabbits than that in normal mucosa, which is consistent with other studies[13,14]. It has been proved that the expression of TFF1 is up-regulated in malignant carcinoma tissues. A hot topic of studies at home and abroad is whether carcinoma leads to the excessive expression of trefoil peptide. In mouse model of TFF1 gene knockout, all gastric epithelial cells showed severe hyperplasia, high dysplasia and adenoma in gastric antrum, which may develop into gastric infiltrative adenocarcinoma[15]. However, the excessive expression of TFF1 in mammal tissues of transgenic rat model does not lead to hyperplasia and dysplasia[16]. TFF1 could restrain proliferation of gastric adenocarcinoma cell line AGS and its inhibition is dose-dependent. TFF1 dimer has a much higher biologic activity than monomer[17], suggesting that TFF1 is a kind of tumor suppression factor. In our experiment, the expression of TFF1 in peripheral tissues of gastric adenocarcinoma was much higher than that in normal gastric mucosa, illustrating that TFF1 is relevant to tumor suppression. The formation of carcinoma promotes the secretion of TFF1 to restrain proliferation of carcinoma, leading to less or no expression of TFF1 in carcinoma tissues. The reasons why the lower the differentiation, the weaker the expression, might be as follows. The decreased expression of TFF1 may lead to formation of carcinoma, and destruction of glands and cells in carcinoma tissues may lead to decreased secretion of TFF1. The poorer the differentiation is, the more seriously the glands and cells are destructed, the less the TFF1 is secreted and expressed. In our study, the expression of TFF1 in moderately-well differentiated adenocarcinoma was a bit lower than that in normal mucosa, but there was no statistically significant difference due to the limited specimens.
At present, more and more attention has been paid to the relationship between precancerous change and carcinogenesis. Intestinalized gastric mucosa and atypical hyperplasia are the most common pathological changes. Our study showed that the expression of TFF1 in atypical hyperplasia was much higher than that in normal mucosa. When the glands of gastric mucosa are not destroyed in early stage of gastric carcinogenesis, the secretion of TFF1 might be promoted to protect gastric mucosa against damage. in intestinalized gastric mucosa, TFF1 was expressed in peripheral tissues of intestinalized mucosa but not in intestinalized gastric. The possible reasons might be as follows. Goblet cells could not express TFF1, and lack of TFF1 induces intestinalization of epithelial cells in gastric mucosa, indicating that TFF1 might play a role in gastric carcinogenesis. Some studies showed that TTF1 can protect mucosa against damage and suppress carcinogenesis, while other studies showed that TTF1 can restrict cell adherence, promote cancer cell invasion, and block necrosis of cancer cells. These findings indicate that TFF1 may be an indicator of metastasis, carcinoma invasion, and poor prognosis[18,19]. Further study is needed to explore the relationship between trefoil peptide and carcinoma.
In addition, no TFF1 is expressed in esophageal carcinoma and its peripheral tissues, and TFF1 is irrelevant to esophageal carcinogenesis and suppression.
S- Editor Liu Y L- Editor Wang XL E- Editor Liu WF
| 1. | Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44:890-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Ribieras S, Tomasetto C, Rio MC. The pS2/TFF1 trefoil factor, from basic research to clinical applications. Biochim Biophys Acta. 1998;1378:F61-F77. [PubMed] [Cited in This Article: ] |
| 3. | Pera M, Heppell J, Poulsom R, Teixeira FV, Williams J. Ulcer associated cell lineage glands expressing trefoil peptide genes are induced by chronic ulceration in ileal pouch mucosa. Gut. 2001;48:792-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932-2938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193-204. [PubMed] [Cited in This Article: ] |
| 6. | Cook GA, Thim L, Yeomans ND, Giraud AS. Oral human spasmolytic polypeptide protects against aspirin-induced gastric injury in rats. J Gastroenterol Hepatol. 1998;13:363-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895-7903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 583] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Chadwick MP, Westley BR, May FE. Homodimerization and hetero-oligomerization of the single-domain trefoil protein pNR-2/pS2 through cysteine 58. Biochem J. 1997;327:117-123. [PubMed] [Cited in This Article: ] |
| 9. | Tanaka S, Podolsky DK, Engel E, Guth PH, Kaunitz JD. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997;272:G1473-G1480. [PubMed] [Cited in This Article: ] |
| 10. | Thim L, Mørtz E. Isolation and characterization of putative trefoil peptide receptors. Regul Pept. 2000;90:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Tan XD, Hsueh W, Chang H, Wei KR, Gonzalez-Crussi F. Characterization of a putative receptor for intestinal trefoil factor in rat small intestine: identification by in situ binding and ligand blotting. Biochem Biophys Res Commun. 1997;237:673-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Newton JL, Allen A, Westley BR, May FE. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut. 2000;46:312-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Ren JL, Lu YP, Wang L, Chen JM, Shi HX, Ye ZH, Wu YH, Hong Y, Lin XT, Lin H. The expression of TFF1 in normal and impaired gastric mucosa. Shijie Huaren Xiaohua Zazhi. 2003;11:1809-1810. [Cited in This Article: ] |
| 14. | Ren JL, Luo JY, Lu YP, Wang L, Chen JM, Shi HX, Pan JS. [Association of trefoil factor 1 expression with gastric mucosa injuries and gastric cancer]. Diyi Junyi Daxue Xuebao. 2005;25:1178-1180. [PubMed] [Cited in This Article: ] |
| 15. | Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, Wendling C, Tomasetto C, Chambon P, Rio MC. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 374] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Tomasetto C, Wolf C, Rio MC, Mehtali M, LeMeur M, Gerlinger P, Chambon P, Lathe R. Breast cancer protein PS2 synthesis in mammary gland of transgenic mice and secretion into milk. Mol Endocrinol. 1989;3:1579-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Calnan DP, Westley BR, May FE, Floyd DN, Marchbank T, Playford RJ. The trefoil peptide TFF1 inhibits the growth of the human gastric adenocarcinoma cell line AGS. J Pathol. 1999;188:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 18. | Yamachika T, Werther JL, Bodian C, Babyatsky M, Tatematsu M, Yamamura Y, Chen A, Itzkowitz S. Intestinal trefoil factor: a marker of poor prognosis in gastric carcinoma. Clin Cancer Res. 2002;8:1092-1099. [PubMed] [Cited in This Article: ] |
| 19. | Dhar DK, Wang TC, Maruyama R, Udagawa J, Kubota H, Fuji T, Tachibana M, Ono T, Otani H, Nagasue N. Expression of cytoplasmic TFF2 is a marker of tumor metastasis and negative prognostic factor in gastric cancer. Lab Invest. 2003;83:1343-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |