Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7233
Revised: July 28, 2006
Accepted: September 20, 2006
Published online: December 7, 2006
Colon cancer is still one of the leading causes of cancer death worldwide. Although the host immune system has been shown to react against tumor cells, mainly through tumor infiltrating lymphocytes and NK cells, tumor cells may utilize different ways to escape anti-tumor immune response. Tumor infiltration of CD8+ and CD4+ (T-bet+) effector T cells has been attributed to a beneficial outcome, and the enhancement of T cell activation through T cell receptor stimulation and co-stimulatory signals provides promising strategies for immunotherapy of colon cancer. Growing evidence supports a role for the Fas/FasL system in tumor immunology, although the mechanisms and consequences of FasL activation in colon cancer are not completely understood. In animal models, depletion of regulatory T cells (CD4+ CD25+ T cells) can enhance the anti-tumor immune response under certain conditions. Taken together, recent insights in the immune reaction against colon carcinoma have provided new approaches to immunotherapy, although much remains to be learned about the exact mechanisms.
- Citation: Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: The role of tumor invading T cells. World J Gastroenterol 2006; 12(45): 7233-7238
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7233.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7233
Colon cancer is still one of the leading causes of cancer death worldwide. In the United States approximately 145 290 new cases of colorectal cancer are diagnosed every year. With more than 56 000 deaths in the United States in 2005, colorectal cancer is responsible for more than 10 percent of all cancer deaths[1]. However, the molecular pathogenesis of colorectal cancer is still poorly understood. Recent studies suggested that different mechanisms such as mutations in cell cycle-[2] and apoptotic-pathways[3], signal transduction[4-6], angiogenesis[7,8], invasion and metastasis[9] significantly contribute to cancer progression (Table 1). Another important mechanism consists of the ability of tumor cells to escape the host immune reaction, as outlined below.
| Pathway | Targets |
| Cell cylce | p53, pRb, p16, p21, D-type cyclins |
| Apoptosis and survival | Bcl-2, Cox-2 |
| Signal transduction | EGF-R, Akt, AP-1, Her2/neu, NFκB |
| Angiogenesis, invasion, and metastasis | VEGF, TSP-1, CXCR-4 |
| Immunity | IL-6, TGF-β |
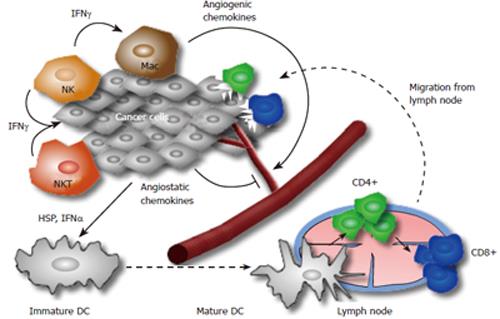
Sir Macfarlane Burnet and Lewis Thomas first proposed the existence of an immunological response to tumors in the cancer immunosurveillance hypothesis in the 1950s[10,11]. However, strong evidence supporting this concept was lacking and the hypothesis was abandoned for many years[12]. In the past two decades, however, the identification of tumor specific antigens and immune modulation leading to tumor regression suggested the existence of cancer immunosurveillance[13-17]. The activation of the host immune system through tumor cells is a complex cascade involving both the innate and adaptive immune systems (Figure 1)[11]. The presence of tumor specific T cells has been correlated with improved clinical outcome in different human cancers[18-21], but does not necessarily result in anti-tumor immunity, since T cells can also promote the progression of tumors through different growth factors[22]. It has been shown that CD8+ T cells and CD4+ effector T cells may have anti-tumor properties, whereas regulatory T cells (CD4+ CD25+ Tregs) may be responsible for immunological hypo-responsiveness observed in cancer[23-26].
The human gastrointestinal tract contains several phenotypically and functionally distinct populations of T cells, which may play a role in anti-tumor immunity[27-29]. Interestingly, T cell activation has been shown in colorectal cancer and proposed as a prognostic factor[30]. The following editorial will discuss recent advantages in our understanding of T cell activation in colorectal cancer and possible therapeutic strategies.
Tumor-infiltrating lymphocytes (TILs) have been isolated from a variety of solid human cancers. It has been widely accepted that one of the most promising T cell subsets for an effective anti-tumor response consists of CD8+ T cells[26]. In a study on 131 patients with colorectal cancer, Naito et al showed a positive correlation between CD8+ T-cells within cancer cell nests and patient survival[30]. Another study using 959 specimens of resected colorectal cancer analyzed the correlation between tumor metastasis and T cell activation[31]. Elevated expression of genes, specific for cytotoxic T lymphocytes (CTLs), such as CD8α, granzyme B, or granulysin as well as Th1-associated genes as T-bet, or interferon-γ was significantly higher in patients without signs of early metastatic invasion as compared to those with early invasion (VELIPI+). In addition, the authors proposed the presence of CD45RO+ CCR7- memory cells as an independent, positive prognostic factor in colorectal cancer. The detected cells represented all subpopulations in the differentiation pathway and are characterized by long-term persistence in vivo and the ability to rapidly expand upon reencounter with antigen[32].
Interestingly, microsatellite instability in colon cancer results in significantly increased infiltration of tumors with larger numbers of CD8+ T cells and is associated with a favorable prognosis[33,34]. Microsatellite instability is characterized by defective DNA mismatch repair mechanisms, which result in higher rates of mutations and cell-to-cell variability in the length of DNA microsatellites. Tumors revealing extensive microsatellite instability, such as Hereditary Nonpolyposis Colorectal Carcinoma (HNPCC), which is associated with germ-line mutations of DNA mismatch repair genes, are designated as microsatellite instability (MSI)-high. In contrast, sporadic colorectal cancers display MSI-H in only 15%[35]. The better prognosis of MSI-H carcinomas is explained by frequent mutations in oncogenic genes and mutation-dependent abnormal peptides resulting in a cytotoxic immune response against cancer cells and hence increased infiltration by CTLs[36]. Several immunogenic antigens, such as mutated CDX2, TGFβIIR, or Caspase-5, have been identified for MSI-H associated colon cancers[37-39]. In MSI-H negative sporadic colorectal carcinoma, the identification of different tumor associated antigens (TAAs) has also been correlated with the activation of the host immune system to tumor cells[34,40-44].
In some patients, recurrent or persistent inflammation may promote carcinogenesis and trigger cancer growth. For instance, the risk of colorectal cancer in patients with chronic inflammatory bowel disease (ulcerative colitis and Crohn’s disease) increases with longer duration of disease[45,46]. In particular, patients with ulcerative colitis have a significant risk for development of colitis associated colon cancer dependent on the extension of the disease, the presence of backwash ileitis and the number of flares suggesting that inflammation drives carcinogenesis. Whereas defined molecular changes in progression of sporadic colon cancer lead to stepwise changes in histology according to the adenoma-carcinoma sequence, carcinogenesis in ulcerative colitis starts with a hyperplastic lesion in the inflamed mucosa and develops through dysplasia into adenocarcinoma, (“inflammation-dysplasia-carcinoma” sequence)[47]. Different molecular mechanisms such as oxidative stress and NF-κB-activation have been attributed to inflammation dependent carcinogenesis[47-49]. Even T cell activation can lead to tumor growth through cytokine secretion. For instance Becker et al showed that IL-6 signaling through TILs results in tumor progression, as IL-6 serves as a growth and proliferation factor for tumor cells. This signaling was dependent on tumor derived soluble IL-6 receptor and could be inhibited through TGF-β[50,51].
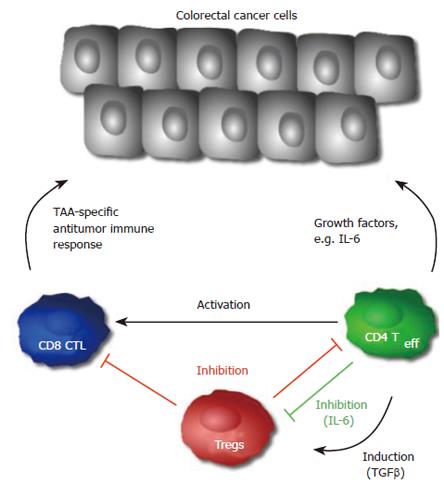
Taken together, the immune system may promote progression of colorectal cancer in cases of chronic inflammation, but can also lead to tumor regression through the innate immune system with tumor specific activation of CTLs and effector CD4+ T cells (Figure 2). However, the high incidence and death rate of colorectal cancer suggest an ineffective immune response in many cases. Although it has been shown that CTLs can recognize specific TAAs, many mechanisms of suppression or failure of such recognition do exist[18,21,52].
It has been proposed that tumor progression is a result of the natural selection of rapidly growing tumor cell variants leading to a growth advantage of such cells over other cells. This concept has also been applied to the immune escape of tumor cells. The loss or down-regulation of HLA classIantigens, the lack of co-stimulation, defective death receptor signaling, apoptosis of activated T cells, immunosuppressive cytokines, and activation of suppressor T cells are important examples for this phenomenon[53].
Norazmi et al described decreased HLA classIanti-gen in malignant colonic tissues as compared to non-malignant tissues from the same individuals[54], thereby enabling tumor cells to escape CTL-mediated lysis due to circumvention of MHC-restricted, antigen-specific triggering of the T-cell receptor (TCR) complex. On the other hand cross-priming of CTLs through antigen presenting cells (APCs) is ineffective, as powerful APC-activating stimuli are usually absent in tumors[53,55].
Antibody-guided targeting of antigenic MHC classI-peptide tetramers on colon cancer cells results in a destruction of tumor cells by cytotoxic T lymphocytes in vitro and in vivo and has been proposed as a new form of immunotherapy[56,57]. Otherwise, data from Luo et al show a protection of colon carcinoma cells from apoptosis and cytolysis induced by hepatic NK cells through higher MHC classIexpression, most likely by blocking the perforin/ granzyme pathway[58].
T cell activation is mediated not only by triggering of the T cell receptor complex, but also by antigen-independent mechanisms such as co-stimulation. Co-stimulation induces cytolysis, cytokine secretion, proliferation and protection from apoptosis in CTLs. The poor immunogenicity of tumor cells has been partly ascribed to the lack of expression of co-stimulatory ligands (see Abken et al[59] for review). In the past few years, the increasing knowledge about the mechanisms of T cell activation led to new approaches for immunotherapy. Concerning colon carcinoma, the therapeutic amplification of the expression of co-stimulatory molecules as B7.1[60] and CD40L[61], the induction of co-stimulatory molecules as OX40 and 4-1BB[62] on T cells, and the administration of soluble co-stimulator proteins as B7.1-Fc[63], a B7.1 fusion protein consisting of the extracellular domains of human B7.1 and the Fc portion of human IgG1, or Ig-4-1BBL[64], a soluble fusion protein of 4-1BB Ligand and IgG2a, have shown promising results in experimental settings.
Growing evidence supports a relevant role of Fas/ Fas Ligand (FasL) interactions in the immune escape of tumors. Fas and FasL belong to the tumor necrosis factor receptor and ligand families and activation of Fas by anti-Fas antibodies results in apoptosis of Fas expressing cells. It has been shown that tumors may provide resistance to Fas-mediated cytotoxicity, and that FasL expression on tumor cells could counterattack the immune system by inducing apoptosis of immune effector cells[65,66].
Several studies gave evidence for a role of the Fas tumor counterattack in colon carcinoma[67-69]. However, in a study with two different Fas-expressing target cell lines and seven different human colon cancer lines Favre-Felix et al failed to detect an induction of apoptosis in Fas-expressing target cell lines, namely Jurkat T cells and murine leukemia cells[70]. Recent studies suggest different functions of FasL in the immune response, since it has been shown that FasL is also delivering costimulatory signals to T cells, inducing motility of tumor cells, contributing to liver regeneration and yielding growth stimulatory signals to neurons[65]. The role of FasL in tumor escape is far from being understood and further studies are mandatory to elucidate the mechanisms and consequences of FasL activation.
While it is generally accepted that CD4+ T cells may contribute to the host anti-tumor immune response, a small subset of CD4+ T cells, the CD4+ CD25+ regulatory T cells (Treg) have been shown to accumulate in the tumor environment and induce immune escape mechanisms[26,71]. Elevated expression of FOXP3, a transcription factor crucial in the development and function of Tregs, has been associated with a poor prognosis in different types of cancer[72,73].
Depletion of Tregs by specific antibodies has enhanced vaccine-induced anti-tumor immunity in colon cancer and other cancer subsets such as leukemia, plasmocytoma, melanoma, fibrosarcoma, or renal cell carcinoma[24,25,74-80]. On the other hand the addition of Tregs resulted in growth regression of inflammation associated intestinal tumors in two studies provided by Erdman et al[81,82]. This is also in agreement with the above-mentioned data about tumor progression through IL-6 signaling, since Tregs can suppress cytokine release. Interestingly enough, TGF-β seems to have a central role in these mechanisms, since the cytokine itself can inhibit IL-6 signaling and lead to FOXP3 expression in tumor infiltrating CD4+ T cells[83]. Accordingly, the role of Tregs in colon carcinoma may also depend on tumor pathogenesis, but the exact mechanisms of Tregs in the regulation of tumor immunology remain undefined.
Colon cancer is still one of the leading causes of cancer death worldwide. Although the host immune system can initiate an immune response against colon cancer cells, tumor cells may utilize different ways to escape those defense mechanisms. Detection of tumor associated antigens, stimulation of the T cell receptor, enhancement of costimulatory signals and depletion of regulatory T cells have shown promising results to overcome tumor escape and provide new strategies for immunotherapy of colon cancer.
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu WF
| 1. | Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294:1255-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Tominaga O, Nita ME, Nagawa H, Fujii S, Tsuruo T, Muto T. Expressions of cell cycle regulators in human colorectal cancer cell lines. Jpn J Cancer Res. 1997;88:855-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Rupnarain C, Dlamini Z, Naicker S, Bhoola K. Colon cancer: genomics and apoptotic events. Biol Chem. 2004;385:449-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130:137-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108:540-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Maeda K, Nishiguchi Y, Kang SM, Yashiro M, Onoda N, Sawada T, Ishikawa T, Hirakawa K. Expression of thrombospondin-1 inversely correlated with tumor vascularity and hematogenous metastasis in colon cancer. Oncol Rep. 2001;8:763-766. [PubMed] [Cited in This Article: ] |
| 8. | Whisenant J, Bergsland E. Anti-angiogenic strategies in gastrointestinal malignancies. Curr Treat Options Oncol. 2005;6:411-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743-1750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1-27. [PubMed] [Cited in This Article: ] |
| 11. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3425] [Cited by in F6Publishing: 3284] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 12. | Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 990] [Cited by in F6Publishing: 946] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 13. | Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 467] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Houghton AN. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 307] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 667] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 16. | Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645-2650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 729] [Cited by in F6Publishing: 747] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 17. | Pagès F, Berger A, Henglein B, Piqueras B, Danel C, Zinzindohoue F, Thiounn N, Cugnenc PH, Fridman WH. Modulation of interleukin-18 expression in human colon carcinoma: consequences for tumor immune surveillance. Int J Cancer. 1999;84:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2457] [Cited by in F6Publishing: 2498] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 19. | Ohtani H. Pathophysiologic significance of host reactions in human cancer tissue: desmoplasia and tumor immunity. Tohoku J Exp Med. 1999;187:193-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 508] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 21. | Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Liénard D, Cerottini JC, Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 419] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 22. | Inagaki A, Ishida T, Ishii T, Komatsu H, Iida S, Ding J, Yonekura K, Takeuchi S, Takatsuka Y, Utsunomiya A. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: high interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int J Cancer. 2006;118:3054-3061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 792] [Cited by in F6Publishing: 820] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 24. | Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211-5218. [PubMed] [Cited in This Article: ] |
| 25. | Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128-3133. [PubMed] [Cited in This Article: ] |
| 26. | Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes. Lab Invest. 2006;86:231-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Viney J, MacDonald TT, Spencer J. Gamma/delta T cells in the gut epithelium. Gut. 1990;31:841-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Fujihashi K, Yamamoto M, McGhee JR, Beagley KW, Kiyono H. Function of alpha beta TCR+ intestinal intraepithelial lymphocytes: Th1- and Th2-type cytokine production by CD4+CD8- and CD4+CD8+ T cells for helper activity. Int Immunol. 1993;5:1473-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Guy-Grand D, Vassalli P. Gut intraepithelial lymphocyte development. Curr Opin Immunol. 2002;14:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491-3494. [PubMed] [Cited in This Article: ] |
| 31. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1553] [Cited by in F6Publishing: 1556] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 32. | Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1387] [Cited by in F6Publishing: 1440] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 33. | Prall F, Dührkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Guidoboni M, Gafà R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macrì E, Lanza G, Boiocchi M. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 35. | Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417-2422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 36. | Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805-1813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 361] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Schwitalle Y, Linnebacher M, Ripberger E, Gebert J, von Knebel Doeberitz M. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004;4:14. [PubMed] [Cited in This Article: ] |
| 38. | Ishikawa T, Fujita T, Suzuki Y, Okabe S, Yuasa Y, Iwai T, Kawakami Y. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003;63:5564-5572. [PubMed] [Cited in This Article: ] |
| 39. | Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93:6-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Suzuki H, Graziano DF, McKolanis J, Finn OJ. T cell-dependent antibody responses against aberrantly expressed cyclin B1 protein in patients with cancer and premalignant disease. Clin Cancer Res. 2005;11:1521-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Koesters R, Linnebacher M, Coy JF, Germann A, Schwitalle Y, Findeisen P, von Knebel Doeberitz M. WT1 is a tumor-associated antigen in colon cancer that can be recognized by in vitro stimulated cytotoxic T cells. Int J Cancer. 2004;109:385-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Campi G, Crosti M, Consogno G, Facchinetti V, Conti-Fine BM, Longhi R, Casorati G, Dellabona P, Protti MP. CD4(+) T cells from healthy subjects and colon cancer patients recognize a carcinoembryonic antigen-specific immunodominant epitope. Cancer Res. 2003;63:8481-8486. [PubMed] [Cited in This Article: ] |
| 43. | Azuma K, Shichijo S, Maeda Y, Nakatsura T, Nonaka Y, Fujii T, Koike K, Itoh K. Mutated p53 gene encodes a nonmutated epitope recognized by HLA-B*4601-restricted and tumor cell-reactive CTLs at tumor site. Cancer Res. 2003;63:854-858. [PubMed] [Cited in This Article: ] |
| 44. | Shawler DL, Bartholomew RM, Garrett MA, Trauger RJ, Dorigo O, Van Beveren C, Marchese A, Ferre F, Duffy C, Carlo DJ. Antigenic and immunologic characterization of an allogeneic colon carcinoma vaccine. Clin Exp Immunol. 2002;129:99-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 46. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 47. | Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 48. | D'Incà R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 857] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 50. | Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 576] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 51. | Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 860] [Cited by in F6Publishing: 812] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 53. | Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3:999-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 743] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 54. | Norazmi M, Hohmann AW, Skinner JM, Bradley J. Expression of MHC class I and class II antigens in colonic carcinomas. Pathology. 1989;21:248-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming. Eur J Immunol. 2003;33:2645-2654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Robert B, Guillaume P, Luescher I, Romero P, Mach JP. Antibody-conjugated MHC class I tetramers can target tumor cells for specific lysis by T lymphocytes. Eur J Immunol. 2000;30:3165-3170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 57. | Donda A, Cesson V, Mach JP, Corradin G, Primus FJ, Robert B. In vivo targeting of an anti-tumor antibody coupled to antigenic MHC class I complexes induces specific growth inhibition and regression of established syngeneic tumor grafts. Cancer Immun. 2003;3:11. [PubMed] [Cited in This Article: ] |
| 58. | Luo D, Vermijlen D, Kuppen PJ, Wisse E. MHC class I expression protects rat colon carcinoma cells from hepatic natural killer cell-mediated apoptosis and cytolysis, by blocking the perforin/granzyme pathway. Comp Hepatol. 2002;1:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Abken H, Hombach A, Heuser C, Kronfeld K, Seliger B. Tuning tumor-specific T-cell activation: a matter of costimulation. Trends Immunol. 2002;23:240-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Sivanandham M, Shaw P, Bernik SF, Paoletti E, Wallack MK. Colon cancer cell vaccine prepared with replication-deficient vaccinia viruses encoding B7.1 and interleukin-2 induce antitumor response in syngeneic mice. Cancer Immunol Immunother. 1998;46:261-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Liu A, Hu P, Khawli LA, Epstein AL. Combination B7-Fc fusion protein treatment and Treg cell depletion therapy. Clin Cancer Res. 2005;11:8492-8502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Xu DP, Sauter BV, Huang TG, Meseck M, Woo SL, Chen SH. The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther. 2005;12:1526-1533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Igney FH, Krammer PH. Tumor counterattack: fact or fiction. Cancer Immunol Immunother. 2005;54:1127-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | O'Connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: cancer as a site of immune privilege. Immunol Today. 1999;20:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 679] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 68. | Zhu Q, Liu JY, Xu HW, Yang CM, Zhang AZ, Cui Y, Wang HB. Mechanism of counterattack of colorectal cancer cell by Fas/Fas ligand system. World J Gastroenterol. 2005;11:6125-6129. [PubMed] [Cited in This Article: ] |
| 69. | Zhang W, Ding EX, Wang Q, Zhu DQ, He J, Li YL, Wang YH. Fas ligand expression in colon cancer: a possible mechanism of tumor immune privilege. World J Gastroenterol. 2005;11:3632-3635. [PubMed] [Cited in This Article: ] |
| 70. | Favre-Felix N, Fromentin A, Hammann A, Solary E, Martin F, Bonnotte B. Cutting edge: the tumor counterattack hypothesis revisited: colon cancer cells do not induce T cell apoptosis via the Fas (CD95, APO-1) pathway. J Immunol. 2000;164:5023-5027. [PubMed] [Cited in This Article: ] |
| 71. | Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 496] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 72. | Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326-8331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 395] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 73. | Ishibashi Y, Tanaka S, Tajima K, Yoshida T, Kuwano H. Expression of Foxp3 in non-small cell lung cancer patients is significantly higher in tumor tissues than in normal tissues, especially in tumors smaller than 30 mm. Oncol Rep. 2006;15:1315-1319. [PubMed] [Cited in This Article: ] |
| 74. | Steitz J, Brück J, Lenz J, Knop J, Tüting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61:8643-8646. [PubMed] [Cited in This Article: ] |
| 75. | Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchianò A, Andreola S. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235-3245. [PubMed] [Cited in This Article: ] |
| 76. | Liu JY, Zhang XS, Ding Y, Peng RQ, Cheng X, Zhang NH, Xia JC, Zeng YX. The changes of CD4+CD25+/CD4+ proportion in spleen of tumor-bearing BALB/c mice. J Transl Med. 2005;3:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 677] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 78. | Li J, Hu P, Khawli LA, Epstein AL. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res. 2003;63:8384-8392. [PubMed] [Cited in This Article: ] |
| 79. | Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borrás-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931-5939. [PubMed] [Cited in This Article: ] |
| 80. | Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267-3275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 81. | Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 82. | Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998-4004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 83. | Becker C, Fantini MC, Neurath MF. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |