Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5569
Revised: April 28, 2006
Accepted: May 22, 2006
Published online: September 14, 2006
Collision tumors are thought to arise from the accidental meeting and interpenetration of two independent tumors. We report here a highly unusual case of a 61-year old man who had a unique tumor that was composed of a metastatic adenocarcinoma from the stomach to the rectum, which harbored a collision tumor of primary rectal adenocarcinoma. The clonalities of the two histologically distinct lesions of the rectal mass were confirmed by immunohistochemical and molecular analysis. Although histologic examination is the cornerstone in pathology, immunohistochemical and molecular analysis can provide evidence regarding whether tumors originate from the same clone or different clones. To the best of our knowledge, this is the first reported case of such an occurrence.
- Citation: Roh YH, Lee HW, Kim MC, Lee KW, Roh MS. Collision tumor of the rectum: A case report of metastatic gastric adenocarcinoma plus primary rectal adenocarcinoma. World J Gastroenterol 2006; 12(34): 5569-5572
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5569
The term collision tumor refers to two coexisting, but independent tumors[1]. Malignant neoplasms originating from two or more distinct topographic organs may form a collision tumor. A possible explanation for this is field cancerization, which occurs due to long-term exposure to carcinogens, whereby multiple carcinogenic transformations give rise to genetically unrelated secondary primary tumors with independent mutations[2,3] and thus, the chance of tumor collision may be increased. However, there is no explanation for the occurrence of many collision tumors. As most diagnoses are made based on the histology alone, the question is whether histologic classification can accurately reflect the molecular findings in these tumors. If two tumors arise independently and are associated with coincidence only, the genetic alterations are expected to be different from each other because of the different tumor origins. We report here a rare collision tumor of the rectum that was composed of a rectal adenocarcinoma within the metastatic gastric adenocarcinoma. Furthermore, in an effort to find the molecular evidence both for the histologic diagnosis of this collision tumor and for the clonality of the two separate components, we characterized the molecular alterations of each tumor component by examining the microsatellite instability (MSI) and loss of heterozygosity (LOH), and performed an immunohistochemical analysis as well. The findings of this tumor represent an entity that has never been described at this location.
A 61-year old man presented with postprandial epigastric pain for 5 mo. Upper gastrointestinal endoscopy suggested an ulcerofungating tumor spreading from the gastric body and antrum to near the esophagogastric junction, and biopsy revealed a poorly differentiated adenocarcinoma. The patient underwent radical total gastrectomy for the gastric cancer with regional lymph node dissection. During the operation, the rectum showed a tumor that was considered to be a distant metastasis from the gastric cancer. So, low anterior resection of the rectum with regional lymph node dissection was also performed for the distant metastasis of the gastric carcinoma.
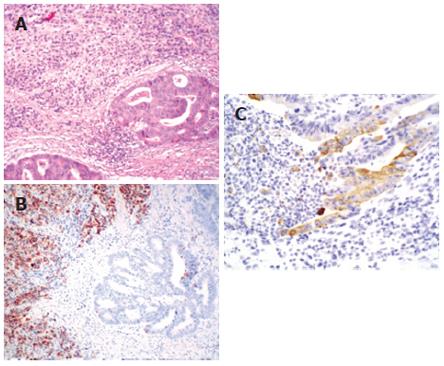
The resected rectum revealed a relatively ill-defined ulcerofungating mass measuring 7 cm × 6 cm. Sectioning revealed a whitish granular infiltrating tumor with extension into the perirectal soft tissue. Any regional differences of the tumor were not grossly identified. The resected stomach revealed a Borrmann type IV mass, measuring 14 cm × 14 cm, at nearly the entire gastric wall. Microscopically, the rectum showed a larger component of poorly differentiated adenocarcinoma with a focal signet-ring cell appearance involving the entire rectal wall. A smaller component of well-differentiated tubular adenocarcinoma invading into the perirectal soft tissue was noted within the poorly differentiated adenocarcinoma. Both components collided with each other with no intermingling at their interface (Figure 1A). The surgical margins were tumor-free. Multiple regional lymph nodes (n = 28) showed metastatic adenocarcinoma (n = 26), of which two revealed feature of well-differentiated tubular adenocarcinoma and 24 revealed poorly-differentiated adenocarcinoma. The stomach mass was an invasive, poorly-differentiated adenocarcinoma invading into the perigastric soft tissue and showing the same histologic features as the larger component of the rectal tumor. The well-differentiated tubular adenocarcinoma cells seen in the rectal wall were not found in the gastric wall. The proximal resection margin was tumor-involved, but the distal resection margin was tumor-free. Multiple regional lymph nodes (n = 60) showed poorly-differentiated metastatic adenocarcinoma (n = 47).
Immunohistochemical staining was performed to distinguish the two components of the rectal tumor. The characteristics of the antibodies used in this study and the results are presented in Table 1 as well as in Figure 1B and 1C. In summary, the primary gastric carcinoma as well as the metastatic gastric carcinoma in the rectum displayed both strong and diffuse staining for MUC2, but negative staining for cytokeratin 7 (CK7), whereas the primary rectal carcinoma component showed focal positive immunoreactivity for CK7, but negative staining for MUC2. The distribution of immunostaining was well correlated with the histologic distinction between metastatic gastric and primary rectal carcinoma components in the collision tumor.
| Immunohisto-chemical markers | Antibody | Results | ||||
| Rectal tumor | Primary stomach | |||||
| Source | Clone | Dilution | Primary rectum | Metastatic stomach | ||
| CK7 | DakoCytomation | OV-TL | 1:200 | Positive | Negative | Negative |
| CK20 | DakoCytomation | Ks20.8 | 1:50 | Positive | Positive | Positive |
| p53 | DakoCytomation | DO-7 | 1:50 | Positive | Positive | Positive |
| MUC2 | Novocastra | Ccp58 | 1:500 | Negative | Positive | Positive |
| MUC5AC | Novocastra | CLH2 | 1:500 | Negative | Negative | Negative |
| CDX2 | Novocastra | CDX2-88 | 1:100 | Positive | Positive | Positive |
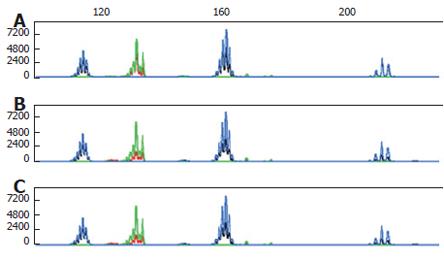
The tissue of both tumors and their non-tumor counterparts were scraped from 10 μm-thick formalin-fixed, paraffin-embedded sections, and then genomic DNA was extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany). DNA sample pairs were amplified using the microsatellite instability MSI/LOH starter kit (Applied Biosystems, Forster City, CA, USA). Genetic stability was analyzed using the Bethesda reference panel that includes BAT25, BAT26, D2S123, D5S346 and D17S250[4]. Both the primary and metastatic gastric carcinoma components as well as the primary rectal carcinoma component showed microsatellite stability (Figure 2). LOH analysis was carried out using 3 polymorphic microsatellite repeat markers including D2S123, D5S346 and D17S250. A value below 0.6 or above 1.6 was interpreted as evidence of LOH, whereas values between these figures were considered retention of heterozygosity. LOH was found in both primary and metastatic gastric carcinoma components, but not in primary rectal carcinoma component (Table 2). Eight months after surgery, the patient died of recurrent gastric cancer.
| Microsatellitemarker | Chromoso-mal region | Tumorsuppressorgene | Loss of heterozygosity | ||
| Rectal tumor | Primarystomach | ||||
| Primaryrectum | Metastaticstomach | ||||
| D2S123 | 2p16 | hMSH2 | No (0.95) | No (0.93) | No (1.03) |
| D5S346 | 5q21 | APC | No (1.31) | Yes (0.50) | Yes (0.54) |
| D17S250 | 17q11.2-12 | P53 | No (1.01) | Yes (0.41) | Yes (0.43) |
Collision tumor is considered as a double tumor showing a ‘side by side’ or ‘one upon another’ pattern. It can occur within the same organ, or in adjacent organs, or in conjunction with systemic malignancy[5]. Several hypotheses have been suggested as the mechanisms for collision tumor. The simplest is that two primary tumors occur in continuity by an accidental “meeting”. Two different tumors may also contiguously develop because the region is altered by the same carcinogenic stimuli. Another hypothesis is that the presence of the first tumor alters the microenvironment, making the development of the second adjacent tumor more likely[5]. In our case, because the primary rectal cancer occupied a smaller portion of the lesion within the larger portion of metastatic gastric cancer, suggesting that metastatic gastric carcinoma to the rectum probably makes some changes in microenvironment and metabolic condition of the rectum, in which a second primary rectal cancer may have developed in association with a previous metastatic focus rather than a metastasis harboring rectal adenocarcinoma. Therefore, we can postulate that substances produced by gastric adenocarcinoma stimulate the immediate adjacent mucosa to undergo increased proliferation and neoplastic transformation.
Collision tumor needs to be distinguished from composite tumor, which is characterized by two divergent lineages originating from the same neoplastic clonal proliferation[1], because different treatments are warranted depending on the type of collision tumor encountered[6]. The behavior of collision tumor depends on the individual elements. Definitive conclusion could not be drawn with regard to the prognosis of this type of collision tumor in our case. However, it is important to differentiate between a case with rectal carcinoma coincidentally having a metastatic gastric carcinoma component and a case with only a primary rectal carcinoma component because of the difference in prognosis. The prognosis of patients with only primary rectal carcinoma is determined by the staging at diagnosis and it is likely to be more favorable than that of patients with an additional metastatic gastric adenocarcinoma in the rectum.
Genetic analysis provides evidence regarding whether the tumors originate from the same clone or from different clones. The panels for each anatomically different site of tumor are then compared to identify the conserved and unique mutations. If the mutational profiles are predominantly unmatched, the diagnosis of a second primary tumor can be established. In an effort to determine the clonality of the two separate components in this patient’s rectal tumor, we characterized the molecular alterations of each tumor component, by MSI and LOH analysis. It has been recently reported that the pattern of MSI findings is a useful tool in determining whether a patient has double primary tumors or a single clonal tumor with metastasis[7]. Kim et al[8] reported that high coincident MSI is observed in 17.7% of patients with colon and stomach cancers, suggesting that a genetic defect of mismatch repair deficiency may be responsible for a small subset of double primary cancers of the colorectum and stomach. In this case, we could not find any discriminating pattern of MSI in both primary and metastatic gastric carcinoma components, as well as in primary rectal carcinoma component. The clonal evolution of cancer can be followed up by using markers that identify LOH. However, carcinogenesis is most often not a single event, but rather the result of many mutations that accumulate over time. Blaker et al[9] reported that LOH patterns of primary colon cancer and metastatic tumors are different in about half of the cases. In our case, LOH analysis showed that the two components of rectal tumor were collision tumor.
The mucin gene family consists of at least nine MUC genes whose tissue distribution has mainly been studied with antibodies that recognize the core protein of different mucins. At immunohistochemical level, the expressed main mucin types are MUC1 for the intestinal type, MUC5AC for the diffuse type, and MUC2 for the mucinous type in gastric cancer[10]. MUC2 expression has been shown to be significantly lower in non-mucinous colorectal cancer[11]. Different expressions of various types of CK in tumors at different primary sites can be a clue to the origin of a neoplasm. It has been reported that carcinomas of the colon generally express CK20, whereas the CK7 expression is usually negative, and the expressions of CK7 and CK20 in carcinomas of the stomach have yielded more variable results[12]. However, carcinomas of a gastrointestinal origin exhibit overlapping and heterogeneous expressions of each mucin and also CK, and there is no definitively consistent immunoreactivity pattern. In this study, primary gastric carcinoma as well as metastatic gastric carcinoma in the rectum displayed strong and diffuse staining for MUC2, but negative staining for CK7, whereas the primary rectal carcinoma component showed focal positive immunoreactivity for CK7, but negative staining for MUC2. The distribution of immunostaining was significantly correlated with the histologic distinction between metastatic gastric and primary rectal carcinoma components in collision tumor.
To the best of our knowledge, although one of the tumors with a significantly elevated risk is colorectal cancer after the diagnosis of stomach cancer, rectal collision tumor with primary rectal adenocarcinoma and metastatic gastric adenocarcinoma is an entity that has not been previously described at this location. Our immunohistochemical and molecular approach clearly demonstrates that the two components of adenocarcinoma of the rectum have a different clonality.
The authors thank SH Jeong of the ISU ABXIS for her molecular technical assistance.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Willis RA. Structure and growth of tumors. Pathology of tumors. 4th ed. London: Butterworth 1967; 138. [Cited in This Article: ] |
| 2. | Eguchi K, Yao T, Konomoto T, Hayashi K, Fujishima M, Tsuneyoshi M. Discordance of p53 mutations of synchronous colorectal carcinomas. Mod Pathol. 2000;13:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Piérard GE, Fazaa B, Henry F, Kamoun MR, Piérard-Franchimont C. Collision of primary malignant neoplasms on the skin: the connection between malignant melanoma and basal cell carcinoma. Dermatology. 1997;194:378-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2154] [Cited by in F6Publishing: 2111] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 5. | Brandwein-Gensler M, Urken M, Wang B. Collision tumor of the thyroid: a case report of metastatic liposarcoma plus papillary thyroid carcinoma. Head Neck. 2004;26:637-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Milne AN, Carvalho R, van Rees BP, van Lanschot JJ, Offerhaus GJ, Weterman MA. Do collision tumors of the gastroesophageal junction exist A molecular analysis. Am J Surg Pathol. 2004;28:1492-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Tang M, Pires Y, Schultz M, Duarte I, Gallegos M, Wistuba II. Microsatellite analysis of synchronous and metachronous tumors: a tool for double primary tumor and metastasis assessment. Diagn Mol Pathol. 2003;12:151-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kim HS, Cho NB, Yoo JH, Shin KH, Park JG, Kim YI, Kim WH. Microsatellite instability in double primary cancers of the colorectum and stomach. Mod Pathol. 2001;14:543-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Bläker H, Graf M, Rieker RJ, Otto HF. Comparison of losses of heterozygosity and replication errors in primary colorectal carcinomas and corresponding liver metastases. J Pathol. 1999;188:258-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 10. | Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Losi L, Scarselli A, Benatti P, Ponz de Leon M, Roncucci L, Pedroni M, Borghi F, Lamberti I, Rossi G, Marino M. Relationship between MUC5AC and altered expression of MLH1 protein in mucinous and non-mucinous colorectal carcinomas. Pathol Res Pract. 2004;200:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Osborn M, van Lessen G, Weber K, Klöppel G, Altmannsberger M. Differential diagnosis of gastrointestinal carcinomas by using monoclonal antibodies specific for individual keratin polypeptides. Lab Invest. 1986;55:497-504. [PubMed] [Cited in This Article: ] |