INTRODUCTION
Acute portal vein thrombosis (PVT) after partial splenic embolization (PSE) in portal hypertension is a rare event[1-3]. Stagnant splenic blood flow after PSE appears to result in PVT[4,5]. Surgical treatment is generally ineffective and yields poor results. Consequently, direct percutaneous intervention is increasing in popularity as a therapeutic alternative. Portal systemic shunts (PSS) are frequently formed due to portal hypertension in patients with chronic liver diseases such as liver cirrhosis or idiopathic portal hypertension (IPH). Balloon-occluded retrograde transvenous obliteration (BRTO) is an effective treatment for gastric varices and encephalopathy with PSS[6-8].
We report a patient with portal vein thrombosis after PSE in IPH with extensive portal systemic shunts treated with transhepatic catheter-directed thrombolysis combined with BRTO restoring portal blood flow.
CASE REPORT
A 66-year-old woman with a medical history of IPH was hospitalized with continuously bloody stool (melena). The HBV and HCV markers were all negative. IPH was diagnosed by liver biopsy and CT scan. Upper endoscopy was performed after admission and small esophageal varices without bleeding were pointed out. Telangiectasia was found in the transverse colon by colonoscopy and hemostasis was accomplished by endoscopic high-frequency coagulation. Despite repeated transfusion of red blood cells and platelets, laboratory examination revealed significant pancytopenia, with a white blood cell count of 1.1 × 109/L, red blood cell count of 2.71 × 1012/L, and platelet count of 2.5 × 1010/L. US and CT revealed splenomegaly.
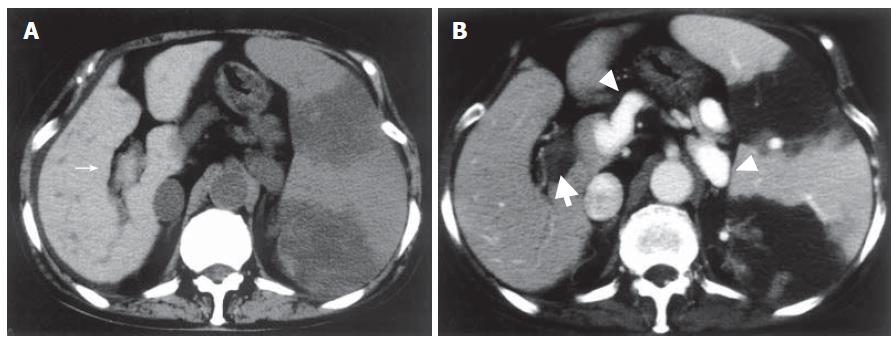
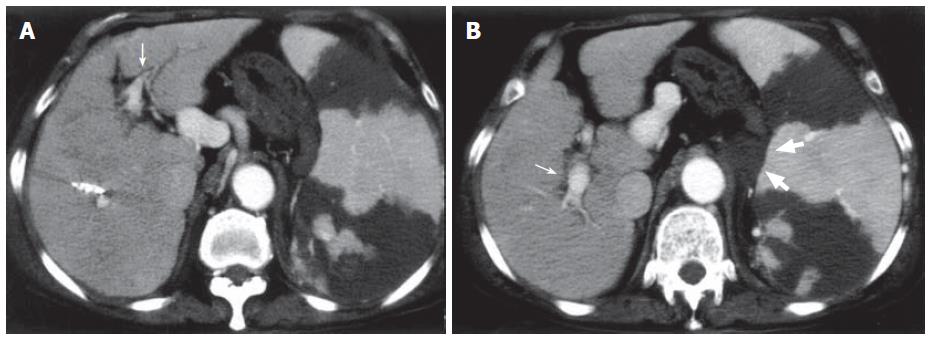
The patient underwent PSE to reduce hypersplenism and portal hypertension. The splenic artery was catheterized selectively via the femoral route, and the tip of microcatheter was inserted in a peripheral branch of the splenic artery. Small particles (1 mm × 1 mm × 1 mm) of gelatin sponge soaked in antibiotics were injected to achieve PSE. The platelet count rose to 14 × 1010/L on the 7th d after PSE. Plain CT revealed a low-density area in the spleen and high-density thrombus in the main portal vein and the 1st right and left branches (Figure 1A), and contrast-enhanced CT revealed no enhancement of the portal vein indicating extensive portal thrombosis (Figure 1B). Portosystemic shunts including a dilated left gastric vein to the paraesophageal vein and splenorenal shunt draining into the left renal vein were also visualized. Thrombolytics and anticoagulants were initiated, urokinase (240 000 U/d) and heparin (10 000 U/d) were given intravenously for 3 d. However, CT after systemic thrombolysis revealed that the thrombosis did not resolve.
Figure 1 Abdominal CT one week after PSE.
A: Plain CT showing high density lesions in the main portal vein and the 1st right branch (narrow arrow); B: Contrast-enhanced CT revealing no enhancement of portal vein indicating portal thrombosis (thick arrow) and portosystemic shunts (arrow head). Splenic infarction after PSE was also visualized.
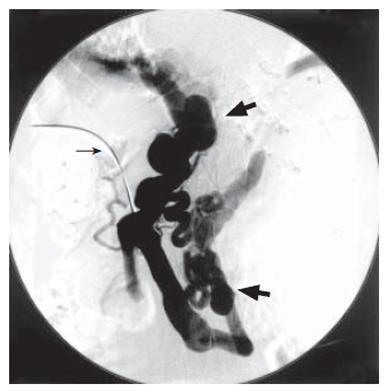
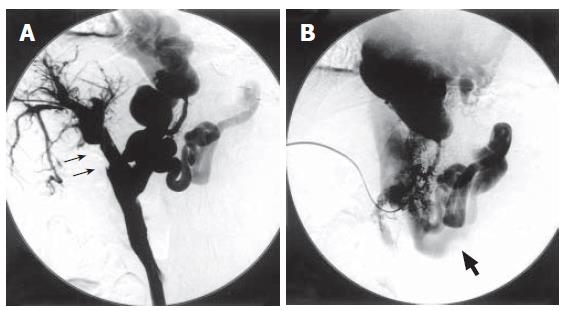
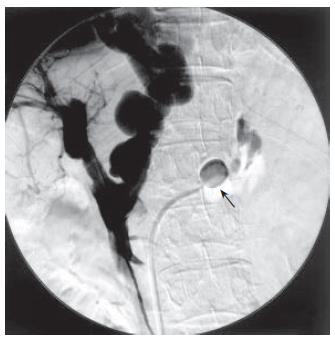
It was therefore decided to attempt catheter-directed thrombolysis. With the patient under local anesthesia, a branch of the right portal vein was punctured with an 18-gauge needle under ultrasonic guidance. A 0.035-inch guidewire was advanced into the portal vein through the thrombus. A 4 Fr. straight catheter (Cook, Bloomington, IN) was advanced into the splenic vein and portography was performed. Percutaneous transhepatic portography (PTP) yielded no visualization of the main portal vein but did show large collaterals of the dilated gastric vein and splenorenal shunt (Figure 2). Urokinase at a dosage of 320 000 U was initially infused directly into the thrombus for 30 min. However, because immediate resolution of the thrombus was not observed, an hourly dose of 20 000 U of urokinase and a daily dose of 10 000 U of heparin were continuously infused into the thrombus. Two days later, PTP revealed total resolution of the thrombus in the portal vein and recirculation of portal blood flow to liver parenchyma (Figure 3A). However, there was still marked hepatofugal flow into the dilated PSS, inducing stagnation of portal blood flow (Figure 3B). BRTO was then attempted to occlude the splenorenal shunt in order to increase portal blood flow. An 8 Fr. J-type sheath (J Sheath; Medikidt, Tokyo, Japan) was inserted into the left renal vein via the right femoral vein. A 7 Fr. balloon catheter (Selecon Cobra-shape MP catheter with 13 mm diameter balloon; Clinical Supply, Gifu, Japan) was advanced through the sheath into the outflow pathway of the splenorenal shunt (Figure 4). Balloon-occluded retrograde venography was carried out to identify the splenorenal shunt and the other outflowing vessels. The amount of sclerosing solution was determined from the volume of contrast material needed to fill these vessels. Preoperatively, 4000 U of haptoglobin (Mitsubishi Pharma, Osaka, Japan) was administered intravenously by drip infusion for the purpose of preventing a renal damage by hemolysis. A total of 10 mL of 5% (50 g/L) ethanolamine oleate iopamidol (EOI) was injected and remained stagnant in these vessels for a day during balloon occlusion. The following day, the balloon catheter was withdrawn after thrombosis of the splenorenal shunt was confirmed on contrast-enhanced CT (Figures 5A and B). The transhepatic catheter was subsequently removed using hemostatic microcoils for the transparenchymal tract without hemorrhagic complications. Six-month follow-up CT revealed good patency in the main portal vein.
Figure 2 No visualization of main portal vein due to extensive thrombosis (narrow arrow) and visualization of portosystemic shunts such as dilated left gastric vein and splenorenal shunt draining into left renal vein (thick arrow) on percutaneous transhepatic portography.
Figure 3 Percutaneous transhepatic portography after catheter-directed thrombolysis.
A: Dissolution of the thrombus in portal vein (narrow arrow); B: Hepatofugal flow into dilated portosystemic shunts (thick arrow: left renal vein).
Figure 4 PTP after BRTO.
BRTO was attempted to occlude splenorenal shunt in order to increase portal blood flow. A 7Fr balloon catheter (arrow) was advanced into the outflow pathway of the splenorenal shunt. A total of 10 mL of 5% ethanolamine oleate iopamidol (EOI) was injected and remained stagnant for a day during balloon occlusion.
Figure 5 Contrast-enhanced CT 1 wk after catheter-directed thrombolysis and BRTO.
A: Good patency of the portal vein (narrow arrow); B: Thrombosed splenorenal shunt (thick arrow).
DISCUSSION
Maddison[9] first reported splenic embolization for hypersplenism in 1973. Spigos et al[10] advocated partial splenic embolization (PSE) as an alternative method for the treatment of portal hypertension in 1979. PSE has been widely used for the treatment of portal hypertension and hypersplenism caused by IPH and liver cirrhosis since then[1-3,9-11]. The aim of PSE is to decrease splenic blood flow and prevent platelet destruction in the spleen. An optimal increase in platelet count could be observed if a single procedure induces an infarct size of at least 60%-80% of the total spleen volume[2,11]. Complications of PSE listed in the literature include local pain, ascites, pleural effusion, splenic abscess and also portal vein thrombosis (PVT) secondary to reduced blood flow in the splenic vein[1-3]. Romano et al[2] reported that no cases of PVT have been observed after PSE in patients with IPH. N’Kontchou et al[3] reported that the incidence of PVT after PSE is 6.3% in patients with cirrhosis. Eguchi et al[4] reported that the incidence of PVT after splenectomy is 25.0% in patients with IPH. PVT can occur with a higher incidence after splenectomy than after PSE.
PVT can occur due to other conditions including liver cirrhosis, IPH, direct invasion of neoplasm, hypercoagulable states, and inflammatory diseases such as pancreatitis and ulcerative colitis[5,12,13]. PVT can result in intestinal ischemia and venous infarction in the acute stage. In the chronic stage, signs of portal hypertension such as esophageal varices, ascites, intestinal bleeding, liver atrophy, and liver failure become prominent[12,13]. Management of acute portal vein thrombosis is thus important. In our case, reduction of splenic blood flow due to PSE and outflow of the superior mesenteric vein (SMV) into the dilated PSS caused portal vein thrombosis.
The standard treatment for PVT is systemic thrombolysis with anticoagulants and local thrombolysis using interventional radiology (IVR) procedures[13]. Urokinase (UK) has been used as a thrombolytic agent for PVT, but an enormous amount of UK is required for systemic use[14], and often has no effect, as in our case. There are two IVR procedures to treat PVT: trans-arterial thrombolysis via the superior mesenteric artery (SMA) and catheter-directed thrombolysis[15]. The angiographic method of trans-arterial thrombolysis is easy and involves only placement of a catheter into the SMA via the femoral artery[16-18]. However, in our patient with remarkable portosystemic collaterals, UK would drain into hepatofugal collaterals and would not reach PVT easily. Local thrombolysis should be more effective for patients with PSS, since high concentrations of UK are directly infused into the PVT through a catheter. Access to catheter-directed thrombolysis is divided into two routes: the transhepatic route[19-21] and the transjugular route[22-24]. Uflacker[24] emphasizes the advantages such as reduction of the possibility of intraabdominal bleeding and creation of adequate portal vein outflow by transjugular intrahepatic portosystemic shunt (TIPS) procedure. However, in our patient with complete portal vein occlusion and large PSS, additional and new creation of a portosystemic shunt can cause complications such as hepatic encephalopathy and hepatic failure[25,26]. We therefore decided to use a transhepatic approach to treat PVT. The transhepatic approach is technically simpler than transjugular approach. This approach allows direct guidewire recanalization of the occluded portion and instillation of high concentrations of UK directly into the thrombus, and reduces the risks of systemic fibrinolysis. In this transhepatic approach there is a risk of uncontrollable bleeding through the transparenchymal tract after removal of the catheter especially in patients with coagulopathy[27]. Avoiding such complications, complete transparenchymal tract embolization with microcoils or gelatin sponge after removal of the catheter is necessary to overcome intraabdominal bleeding. In our case, the transparenchymal tract was embolized with microcoils, and PVT was dissolved completely by transhepatic catheter-directed thrombolysis without hemorrhagic complications. We believe that local thrombolytic therapy via transhepatic route should be considered as a feasible, efficacious and safe treatment.
In the present case, even after recanalization of the portal vein, the patient had stagnancy of portal blood flow with outflow of the SMV into the large PSS. Unless the blood flow to PSS is decreased to increase the portal blood flow, PVT may recur after dissolution. Concurrent BRTO was therefore performed to occlude the splenorenal shunt in our patient, and achieved long-term patency of the portal vein. There is no previous report describing the necessity for concurrent occlusion of portosystemic shunt after recanalization of PVT.
In conclusion, catheter-directed thrombolysis combined with BRTO is feasible and useful for recanalizing PVT and maintaining stable patency of the portal vein in patients with PVT.