Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 21, 2006; 12(27): 4281-4295
Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4281
Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4281
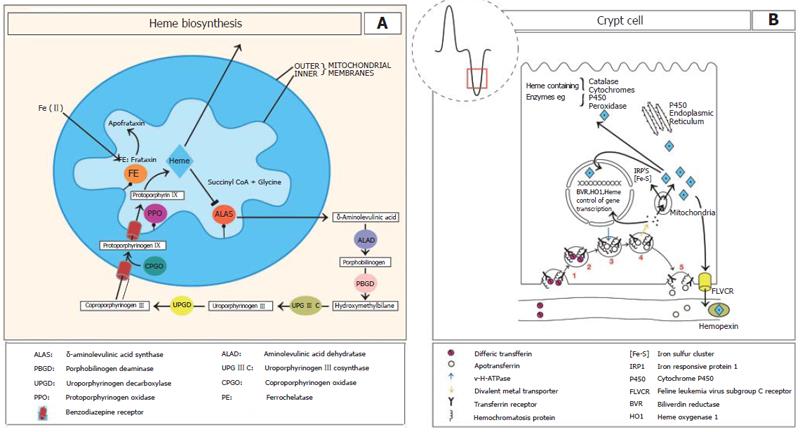
Figure 1 A: The heme biosynthetic pathway.
Mitochondrial and cytosolic locations of the eight enzymes are shown circled and coloured. Commencing synthesis is ALAS on the inner mitochondrial membrane of the first intermediate as well as subsequent intermediates. Heme synthesis is regulated by heme at the level of ALAS via feedback repression. It has been suggested that frataxin may donate ferrous iron to protoporphyrin in the formation of heme; B: In the intestinal crypts the uptake of plasma transferrin-iron occurs by the transferrin receptor (TfR). In iron deficiency HFE complexes with TfR1 and to a much lesser extent with iron loading. (1) TfR binds to plasma diferric transferrin. (2) TfR is internalised by receptor mediated endocytosis. (3) In the cytoplasm a v-H+ATPase fuses with the endosome and acidifies it to release the iron from transferrin. Following ferrireduction Fe(II) is transported to the cytoplasm by a metal transporter. (4) possibly divalent metal transporter 1 (DMT1). The iron is then transported into the mitochondria where it is incorporated into heme. The mitochondria are also a major producer of iron sulphur clusters. (5) The transferrin receptor - apotransferrin complex then return to the cell membrane where at the neutral pH, apotransferrin dissociates. Heme, heme oxygenase and BVR may regulate gene transcription during enterocyte differentiation. FLVCR functions to export excess heme.
- Citation: Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol 2006; 12(27): 4281-4295
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4281