Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4304
Revised: April 28, 2005
Accepted: April 30, 2005
Published online: July 21, 2006
AIM: To investigate the changes that occur in E-cadherin expression during the process of metastasis in colorectal cancer.
METHODS: E-cadherin expression was detected by immunohistochemistry and two indices of expression were calculated which reflected the level of expression and the locations (membrane and cytoplasm). Univariate and multivariate survival analyses were used to assess the value of these two E-cadherin indices as predictors of both disease-free (DFS) and disease-specific (DSS) survival.
RESULTS: E-cadherin membrane index (MI), but not cytoplasmic index (CI), was significantly higher in primary tumors than their metastases (P = 0.0001). Furthermore, both primary tumor MI and CI were higher among the patients who developed subsequent metastasis (P = 0.022 and P = 0.007, respectively). Interestingly, both indices were higher in liver metastase compared to other anatomic sites (MI, P = 0.034 and CI, P = 0.022). The CI of the primary tumors was a significant predictor of DFS (P = 0.042, univariate analysis), with a strong inverse correlation between CI and DFS (P = 0.006, multivariate analysis). Finally, the MI of primary tumor proved to be a significant independent predictor of DSS, with higher indices being associated with a more favorable outcome (P = 0.016).
CONCLUSION: Examination of E-cadherin expression and distribution in colorectal tumors can be extremely valuable in predicting disease recurrence. The observation that aberrant cytoplasmic expression of E-cadherin can predict disease recurrence is obviously of great importance for both patients and clinicians, and significantly affects decisions concerning the therapy and management of the patients.
- Citation: Elzagheid A, Ålgars A, Bendardaf R, Lamlum H, Ristamaki R, Collan Y, Syrjanen K, Pyrhonen S. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol 2006; 12(27): 4304-4309
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4304.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4304
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in industrialized Western countries. The prognosis of CRC patients dramatically depends on the development of recurrence and/or metastasis. However, one of the major problems is the definition of reliable criteria for predicting recurrence and identifying the tumors that will respond to chemotherapy[1,2].
The metastatic cascade starts with a breakdown of the epithelial integrity, which enables tumor cells to invade the surrounding stroma, penetrate either into blood or lymphatic vessels, and finally infiltrate into the appropriate target organs. Epithelial differentiation is critically dependent on maintenance of intact intercellular junctions by cell-cell adhesion molecules. Impairment of these junctions facilitates the invasion of epithelial cells, thus favoring the progression of carcinomas[3]. There are several cell-cell adhesion molecules, including cadherins (E-, P-, N-cadherins), catenins (α, β, and γcatenins), and the CD44 family (standard and variants)[4-6].
Of these cell-cell adhesion molecules, E-cadherin is normally located at the zonula adherens and is functionally necessary for epithelial integrity[7,8]. Decreased levels of E-cadherin expression were reported in many immunohistochemical studies on epithelial malignancies[9-11]. In some tumor types, including CRC, the loss of E-cadherin expression is associated with the loss of tumor differentiation and is shown to be correlated with an increased likelihood of distant metastasis. These data suggest an important role of E-cadherin in tumor invasion and/or slowing down metastases[12-14].
In the present study, we hope to extend these studies using immunohistochemistry to examine the levels of E-cadherin expression in primary tumors and their metastases from a series of 42 CRC patients. E-cadherin expression on the cell membrane and its cytoplasmic accumulation were assessed separately. Univariate and multivariate survival analyses were used to determine the potential predictive and prognostic value of E-cadherin expression in these patients.
A series of 42 patients were diagnosed and treated for Duke’s B, C and D CRC at the Department of Oncology and Radiotherapy, Turku University Central Hospital and six other hospitals of the same hospital district between January 1996 and August 2003. The key clinical characteristics of the patients are summarized in Table 1. The patients were prospectively followed up until death or when they were seen alive for the last time, with a mean follow-up time of 32.3 mo (median 28.2) for the whole series in this study. At diagnosis, 30 patients had metastasis and the remaining patients developed metastatic diseases during follow-up. Disease-specific survival (DSS) and disease-free survival (DFS) were calculated based on the time from diagnosis to death (due to disease), and on the time from diagnosis to the appearance of metastatic diseases, respectively. In calculating the DSS, the patients who died from other or unknown causes were treated as censored cases.
| Variables | n (%) |
| Patients | 42 (100) |
| Female | 25 (59.5) |
| Male | 17 (40.5) |
| Primary tumor status (T) | |
| T1 | 0 (0.0) |
| T2 | 1 (2.4) |
| T3 | 35 (83.3) |
| T4 | 6 (14.3) |
| Primary nodal status (N) | |
| N0 | 19 (47.5) |
| N1 | 21 (52.5) |
| Duke’s staging | |
| B | 9 (21.4) |
| C | 9 (21.4) |
| D | 24 (57.1) |
| Histological grade | |
| I | 9 (21.4) |
| II | 27 (64.3) |
| III | 6 (14.3) |
| Site of metastasis | |
| Local | 6 (14.3) |
| Liver | 13 (31.0) |
| Ovary | 3 (7.1) |
| Peritoneum | 3 (7.1) |
| Mesentery | 4 (9.5) |
| Omentum | 6 (14.3) |
| Lymph node | 2 (4.8) |
| Other1 | 5 (11.9) |
Formalin-fixed, paraffin-embedded samples were available from all 42 primary tumors and their metastases. Paraffin blocks were cut on polylysin-coated microscopy slides for immunohistochemistry. The sections were deparaffinized in xylene, thrice for 10 min, then dehydrated in a descending series of ethanol (100%, 96%, 70%), followed by washes in TBS (0.05 mmol/L Tris-buffer physiological saline, pH 7.4-7.6), thrice for 5 min. Antigen retrieval was achieved by heating the samples without boiling in 10 mmol/L sodium citrate buffer, pH 6.0 (200 mL) in a microwave oven. This treatment was conducted twice for 7 min. The sections were washed in TBS buffer for 30 min. The endogenous peroxide was blocked by 0.3% hydrogen peroxide in methanol for 20 min. The sections were washed in TBS for 15 min.
To inhibit non-specific binding, the samples were incubated in diluted normal horse serum (15 μL serum per 1 mL of TBS-buffer), in humid boxes for 30 min. The primary antibody was monoclonal mouse E-cadherin antibody (clone HECD-1, sub-class IgG1, Zymed Laboratories, San Francisco, CA, USA), at a dilution of 1:300 from the stock. The dilution was based on dilution experiments. Bound primary antibody was visualized with the avidin-biotin peroxidase technique (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA). The antibody was diluted with 20 mmol/L TBS, pH 7.4 (10 mmol/L CaCl2, 0.1% NaN3 and 1% BSA). The sections were incubated in the diluted antibody and the negative controls in 1% BSA in TBS-buffer. The antibody and the 1% BSA incubations were done with 195 μL of the incubation fluid, which was enough to cover the whole section. The incubation took place in incubation boxes at 4°C overnight. After the incubation, the boxes containing sections were left at room temperature for 30 min. The incubation fluid (primary antibody or bovine albumin) was washed off with TBS-buffer (two washes). The secondary antibody (4.5 μL biotinylated anti-mouse antibody in 1 mL of 1% BSA) was pipetted onto the sections and incubated in the moist box for 30 min. The secondary antibody was washed in TBS buffer for 15 min. Vectastain ABC reagent was then used to stain the fixed secondary antibody. After 30 min of incubation, the sections were washed with TBS-buffer.
The final staining was done in diaminobenzidine tetrahydrochloride (DAB) solution (49 mL TBS-buffer, 34 mg imidazole, 17 μL 30% hydrogen peroxide and 1 mL 30% DAB), for 5 min. The slides were washed with distilled water, 70% ethanol for 1 min, then in distilled water. The nuclei were stained with Mayer’s hematoxylin for 30 s. Extra stain was washed with tap water. The slides were then transferred through ascending ethanol series, and xylene before mounting.
The E-cadherin staining was evaluated by an observer blinded to the clinical data (AE) under regular light microscopy. Only the infiltrating part of the neoplasm was evaluated. Both the membranous and cytoplasmic staining were evaluated separately. For cell membrane staining, four categories were used (+++, ++, +, -), starting from equivalent to normal through to entirely negative[10]. The cytoplasmic staining was also graded into four categories: (0) negative, no detectable staining, (1) weak, but still detectable staining, (2) moderate, clearly positive but still weak, and (3) heavy staining, intense. In calculating the staining index; membrane (MI) and cytoplasmic (CI), both the intensity of staining and the fraction of positively stained cells were taken into account, using the following formula:
Ι = 0*f0 + 1*f1 + 2*f2 + 3*f3
where, I is the staining index, f0-f3 the fractions of the cells showing a defined level of staining intensity (from 0 to 3). Theoretically, the index could vary between 0 and 3[15]. The reproducibility of the E-cadherin staining indices was tested twice by one of the observers (AE) analyzing the sections, after a few days (intra-observer variation).
Statistical analyses were made using SPSS® (SPSS, Inc., Chicago, USA) and STATA (Stata Corp., TX, USA) software packages (SPSS for Windows, version 12.0.1 and STATA/SE 8.2). Frequency tables were analyzed using the χ2 test, and likelihood ratio (LR) or Fisher’s exact test was used to assess the significance of the correlation between the categorical variables. Odds ratios and their 95% confidence intervals (95%CI) were calculated where appropriate by the exact method. Differences in the means of continuous variables were analyzed using non-parametric tests (Mann-Whitney or Kruskal-Wallis) for 2- and K-independent samples, respectively. Analysis of variance (ANOVA) was only used for deriving the mean values and their SD of each individual category. Bivariate correlation (Spearman rho) and scatter plots were used to check the correlations between the two continuous variables (MI, CI vs DFS, DSS), controlled by linear regression analysis (R and R2) for linearity. Univariate survival (life-table) analysis for the outcome measure (DSS, DFS) was based on Cox’s method (indices treated as continuous variables). Multivariate survival analysis was made using Cox’s proportional hazards model in a backward stepwise manner with the log-likelihood ratio (L-R) significance test, and using the default values for enter and exclusion criteria. The assumption of proportional hazards was controlled by log-minus-log (LML) survival plots. In all tests, the values P < 0.05 were regarded statistically significant.
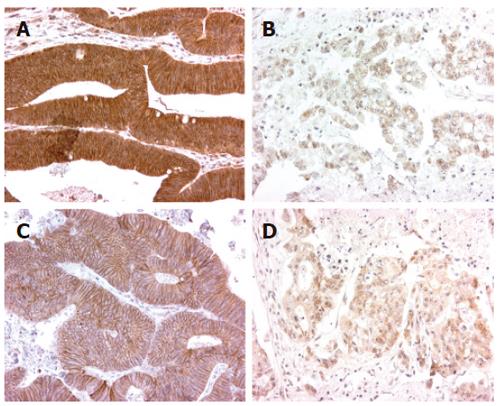
E-cadherin expression was assessed in all tumors (Figure 1). The expression pattern of E-cadherin was predominantly membranous in normal colonic epithelium but this pattern was disturbed (diffuse cytoplasmic and membranous) in the tumor area.
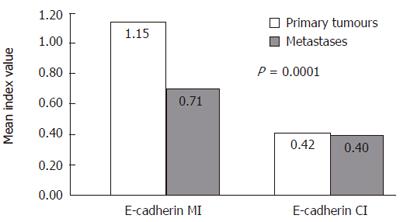
E-cadherin indices in primary tumors and metastases are shown in Figure 2. Interestingly, MI was significantly higher in primary tumors compared with their metastases (P = 0.0001), whereas CI did not show such difference. Both membrane and cytoplasmic E-cadherin expression in the primary tumor was increased in patients who developed metastases (P = 0.022 and P = 0.007, respectively).
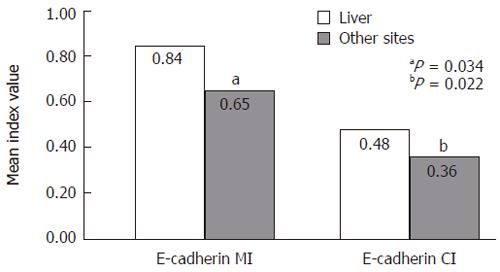
Among the metastatic lesions, MI and CI were related to the site of metastases as shown in Figure 3, both indices were higher in metastases of the liver than in those at other anatomic sites (P = 0.034 and P = 0.022, respectively.)
E-cadherin CIs were significantly higher in the primary tumors from patients who were above the median age of 67.5 years compared with those who were below (P = 0.044). MI and CI did not significantly correlate with other clinical variables such as sex, tumor grade, tumor stage, etc.
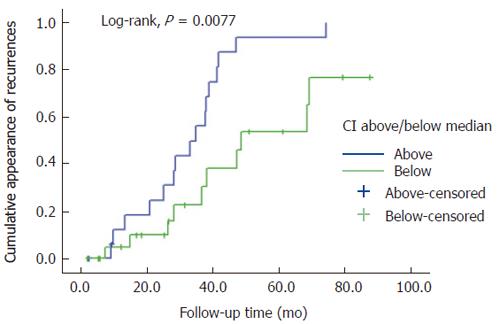
The Cox model was used to analyze the power of E-cadherin indices as predictors of DFS and DSS, using univariate mode for each of the values separately, followed by the multivariate test of all index values entered in the model. In univariate analysis, only the CI in the primary tumors was a significant predictor of DFS, with OR of 3.25 (95%CI 1.04-10.13) (P = 0.042). When all indices were entered in the multivariate Cox model, the CI of the primary tumor retained its value as an independent predictor of DFS, with OR of 4.65 (95%CI 1.36-15.89), while the others dropped out from the model. Indeed, there was a strong inverse correlation between CI and DFS, as shown in the scatter plot (Figure 4), cytoplasmic expression in the primary tumor was significantly higher in patients with disease recurrence (n = 26) during the follow-up period than those without (R2 linear = -0.126, and r = 0.354 in linear regression analysis, as well as in bivariate correlation) (Spearman rho = -0.418; P = 0.006). This independent predictive power of the CI was not confounded by lymph node status, tumor location, primary T, grade, or age (continuous or dichotomized). In fact, predictive power increased in the final model to OR 7.30 (95%CI 1.51-35.10) (P = 0.013).
In the analysis for DSS, none of the indices in either the primary tumor or the metastasis were significant predictors of DSS in the univariate mode. However, in the multivariate model, adjusted for all recorded variables, MI in the primary tumor proved to be a significant independent predictor of survival, in that the higher indices were associated with a more favorable outcome, OR 0.332 (95%CI 0.136-0.813) (P = 0.016). In scatter plot, R2 linear was 0.058 and in linear regression, r = 0.240 (Spearman rho = 0.267, P = 0.091). None of the other variables in the model proved to be significant independent predictors of DSS.
Cancer metastasis occurs through various steps and many biological factors are involved in these processes[16]. Among these, the adhesion molecules are closely involved in the development and growth of metastatic tumors[17]. If the activity of adhesion molecules is decreased, cells tend to detach from their site of origin. Conversely, the reattachment of tumor cells at the sites of metastases is mediated by multiple adhesion molecules, including integrins, selectins and cadherins[18].
Colorectal epithelial cells of the crypt normally exhibit strong membranous expression of E-cadherin at cell-cell borders. This reflects the normal localization of this intercellular adhesion molecule[19]. When we assessed the level and pattern of expression of E-cadherin in primary colorectal tumors and their metastases, membranous and cytoplasmic expression at both locations was consistent with the previous reports[20-23]. Multiple mechanisms are known to cause abnormal E-cadherin expression, including mutations or deletions of the E-cadherin gene itself, mutations in the β-catenin gene, and transcriptional repression of the E-cadherin gene, e.g., by hypermethylation or chromatin rearrangements in the E-cadherin promoter region[24,25]. It is not yet clear which of these are relevant in CRC. However, in this study, the presence of both membranous and cytoplasmic expression in some tumors suggests that the E-cadherin molecule is still capable of locating the membrane, but its abnormal presence in the cytoplasm may be due to other reasons.
In this study, primary tumors showed significantly higher membranous expression of E-cadherin compared with the metastases, while cytoplasmic expression did not differ between the two tumor groups. This may reflect the selection of cells with less E-cadherin expression as metastases are formed, or possibly the local environment of the organ in which the metastasis has been formed. Our findings suggest that the site of metastasis is associated with the distribution of E-cadherin expression. There were differences in E-cadherin expression (both CI and MI) among the metastatic lesions at different anatomic locations. Both MI and CI were significantly higher in metastatic lesions in the liver than in metastasis at other sites (Figure 3). Although these observations implicate several possible mechanisms, there is little data to draw any definitive conclusions; however, Bongiorno et al reported that cancer cells in thoracic neoplasms and in Barrett’s esophagus lost their E-cadherin expression transiently during their dissemination, and then re-expressed this and other adhesion molecules at a distant metastatic site[26]. This concept is indirectly supported by the present observations that MI was significantly higher in primary tumors than in their metastases, whereas CI did not show such a difference. Our findings justify the need to examine the expression of other adhesion molecules so as to investigate the possibility that circulating cancer cells with a distinct adhesion molecule pattern would be preferentially selected to deposit in the liver. Some debate exists concerning the changes in the level of E-cadherin expression in the primary CRC and its metastasis. Gagliardi and co-workers compared 14 liver metastases with their corresponding primary tumors and found complete loss of membranous E-cadherin expression in 7/14 (50%) liver metastases of which 6/7 (86%) showed intense expression in the corresponding primary tumors[19], while Ikeguchi and colleagues reported increased E-cadherin expression in up to 40% of the liver metastases compared with only 17% of the metastatic lymph nodes that were studied[27]. Similarly, Mayer et al reported that in many gastric carcinomas, E-cadherin expression in metastatic lesions of the liver was indeed stronger than that in the primary tumors[28].
When we looked at the patients with metastases at the time of diagnosis (n = 30), we found that CI was almost double in their primary tumors, suggesting that increased aberrant distribution of E-cadherin in the primary tumors may reflect an increased likelihood of metastasis formation. Furthermore, cytoplasmic expression in the primary tumors was significantly higher in patients with disease recurrence (n = 26) during the follow-up period than those without recurrence. The E-cadherin MI was very similar among those who had metastasis at onset and in those who developed recurrence during follow-up (1.13 vs 1.25, respectively). In addition, CI values in the primary tumors of patients who had primary metastasis (0.48) were very similar to those who had subsequent recurrence (0.54). In both cases, CI was always lower among those without primary metastasis and in those who did not develop recurrent disease, (0.27 and 0.22, respectively). These findings suggest that cytoplasmic (aberrant) E-cadherin expression more closely reflects the adverse biological behavior of CRC than does membranous (normal) expression.
In univariate analysis, the cytoplasmic E-cadherin index in primary tumors was a significant predictor of DFS, and CI retained its independent predictive power of DFS also in the multivariate model. In fact, its predictive power substantially increased in the final multivariate model. This effect is explained by the strong inverse correlation between CI levels and DFS (Figure 4). The CI did not predict DSS, however, in either univariate or multivariate model. Interestingly, the MI of the primary tumor proved to be a significant predictive factor in multivariate analysis (with OR and 95%CI far below 1.0), indicating that higher MI values are associated with more favorable disease outcome, suggesting that membranous (normal) E-cadherin expression may reflect a tumor that retains a more normal cell phenotype.
Certainly, our findings support the concept that aberrant (cytoplasmic) E-cadherin expression is a sign of adverse disease outcome in CRC. These results are consistent with the previous reports that loss of normal expression of E-cadherin is associated with poor prognosis in breast and colorectal carcinoma[29,30]. The present study further confirms the power of E-cadherin indices as predictors of disease recurrence and progression.
This study found no significant correlation between E-cadherin indices and the various clinical variables recorded (sex, tumor grade, tumor stage, etc); however, this is not surprising since other groups have also reported similar findings[31].
Finally, the importance of distinguishing between membrane and cytoplasmic E-cadherin expression is emphasized by this study, because it seems to reflect different aspects of the behavior of CRC. Cytoplasmic expression more closely predicts disease recurrence and aberrant expression (downregulation) at the membrane seems to indicate a poorer prognosis. In both cellular locations, E-cadherin expression is reduced in metastasis as compared with the primary tumors. We can only speculate at the mechanisms of this change in expression, and it may be due to alterations in catenins, which link the cadherin molecules to the actin cytoskeleton. Similarly, the observed higher levels of E-cadherin expression (both MI and CI) in metastatic deposits in the liver than in other sites need further investigations.
To conclude, it is clear that examining E-cadherin expression and distribution in colorectal tumors is extremely valuable in predicting disease recurrence. The observation that aberrant cytoplasmic expression of E-cadherin can predict disease recurrence is obviously of great importance for both the patient and the clinician, and affects significantly decisions concerning the therapy and management of the patients.
The skilful technical assistance of Ms. Sinikka Kollanus in preparing sections for IHC is gratefully acknowledged.
S- Editor Guo SY L- Editor Ma JY E- Editor Ma WH
| 1. | Hawk ET, Limburg PJ, Viner JL. Epidemiology and prevention of colorectal cancer. Surg Clin North Am. 2002;82:905-941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Mandelson MT, Miglioretti D, Newcomb PA, Harrison R, Potter JD. Hormone replacement therapy in relation to survival in women diagnosed with colon cancer. Cancer Causes Control. 2003;14:979-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Birchmeier W, Weidner KM, Hülsken J, Behrens J. Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Semin Cancer Biol. 1993;4:231-239. [PubMed] [Cited in This Article: ] |
| 4. | Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169-1180. [PubMed] [Cited in This Article: ] |
| 5. | Lin YC, Wu MY, Li DR, Wu XY, Zheng RM. Prognostic and clinicopathological features of E-cadherin, alpha-catenin, beta-catenin, gamma-catenin and cyclin D1 expression in human esophageal squamous cell carcinoma. World J Gastroenterol. 2004;10:3235-3239. [PubMed] [Cited in This Article: ] |
| 6. | Fernández JC, Vizoso FJ, Corte MD, Gava RR, Corte MG, Suárez JP, García-Muñíz JL, García-Morán M. CD44s expression in resectable colorectal carcinomas and surrounding mucosa. Cancer Invest. 2004;22:878-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 751] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 8. | Zbar AP, Simopoulos C, Karayiannakis AJ. Cadherins: an integral role in inflammatory bowel disease and mucosal restitution. J Gastroenterol. 2004;39:413-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lipponen P, Saarelainen E, Ji H, Aaltomaa S, Syrjänen K. Expression of E-cadherin (E-CD) as related to other prognostic factors and survival in breast cancer. J Pathol. 1994;174:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Elzagheid A, Kuopio T, Ilmen M, Collan Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: the methodology and clinical relevance. Histopathology. 2002;41:127-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217-R222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Sanders DS, Perry I, Hardy R, Jankowski J. Aberrant P-cadherin expression is a feature of clonal expansion in the gastrointestinal tract associated with repair and neoplasia. J Pathol. 2000;190:526-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Keleg S, Büchler P, Ludwig R, Büchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Bendardaf R, Elzagheid A, Lamlum H, Ristamäki R, Collan Y, Pyrhönen S. E-cadherin, CD44s and CD44v6 correlate with tumour differentiation in colorectal cancer. Oncol Rep. 2005;13:831-835. [PubMed] [Cited in This Article: ] |
| 15. | Lipponen P, Collan Y. Simple quantitation of immuno-histochemical staining positivity in microscopy. Acta Stereol. 1992;11:125-132. [Cited in This Article: ] |
| 16. | Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 532] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Lukas Z, Dvorak K. Adhesion molecules in biology and oncology. Acta Vet Brno. 2004;73:93-104. [Cited in This Article: ] |
| 18. | Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 671] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 19. | Gagliardi G, Kandemir O, Liu D, Guida M, Benvestito S, Ruers TG, Benjamin IS, Northover JM, Stamp GW, Talbot IC. Changes in E-cadherin immunoreactivity in the adenoma-carcinoma sequence of the large bowel. Virchows Arch. 1995;426:149-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Nawrocki B, Polette M, Van Hengel J, Tournier JM, Van Roy F, Birembault P. Cytoplasmic redistribution of E-cadherin-catenin adhesion complex is associated with down-regulated tyrosine phosphorylation of E-cadherin in human bronchopulmonary carcinomas. Am J Pathol. 1998;153:1521-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Pedersen KB, Nesland JM, Fodstad Ø, Maelandsmo GM. Expression of S100A4, E-cadherin, alpha- and beta-catenin in breast cancer biopsies. Br J Cancer. 2002;87:1281-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Li YJ, Ji XR. Relationship between expression of E-cadherin-catenin complex and clinicopathologic characteristics of pancreatic cancer. World J Gastroenterol. 2003;9:368-372. [PubMed] [Cited in This Article: ] |
| 23. | Zhang HK, Zhang QM, Zhao TH, Li YY, Yi YF. Expression of mucins and E-cadherin in gastric carcinoma and their clinical significance. World J Gastroenterol. 2004;10:3044-3047. [PubMed] [Cited in This Article: ] |
| 24. | Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 653] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 25. | Goodsell DS. The molecular perspective: cadherin. Oncologist. 2002;7:467-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Bongiorno PF, al-Kasspooles M, Lee SW, Rachwal WJ, Moore JH, Whyte RI, Orringer MB, Beer DG. E-cadherin expression in primary and metastatic thoracic neoplasms and in Barrett's oesophagus. Br J Cancer. 1995;71:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Ikeguchi M, Taniguchi T, Makino M, Kaibara N. Reduced E-cadherin expression and enlargement of cancer nuclei strongly correlate with hematogenic metastasis in colorectal adenocarcinoma. Scand J Gastroenterol. 2000;35:839-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690-1695. [PubMed] [Cited in This Article: ] |
| 29. | Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105:394-402. [PubMed] [Cited in This Article: ] |
| 30. | Aoki S, Shimamura T, Shibata T, Nakanishi Y, Moriya Y, Sato Y, Kitajima M, Sakamoto M, Hirohashi S. Prognostic significance of dysadherin expression in advanced colorectal carcinoma. Br J Cancer. 2003;88:726-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Khoursheed MA, Mathew TC, Makar RR, Louis S, Asfar SK, Al-Sayer HM, Dashti HM, Al-Bader A. Expression of E-cadherin in human colorectal cancer. Surgeon. 2003;1:86-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |