Published online Jun 21, 2006. doi: 10.3748/wjg.v12.i23.3707
Revised: December 28, 2005
Accepted: January 9, 2006
Published online: June 21, 2006
AIM: To evaluate the treatment effect of percutaneous ethanol injection (PEI) for patients with advanced, non-resectable HCC compared with combination of transarterial chemoembolisation (TACE) and repeated single-session PEI, repeated single-session PEI alone, repeated TACE alone, or best supportive care.
METHODS: All patients who received PEI treatment during the study period were included and stratified to one of the following treatment modalities according to physical status and tumor extent: combination of TACE and repeated single-session PEI, repeated single-session PEI alone, repeated TACE alone, or best supportive care. Prognostic value of clinical parameters including Okuda-classification, presence of portal vein thrombosis, presence of ascites, number of tumors, maximum tumor diameter, and serum cholinesterase (CHE), as well as Child-Pugh stage, α-fetoprotein (AFP), fever, incidence of complications were assessed and compared between the groups. Survival was determined using Kaplan-Meier and multivariate regression analyses.
RESULTS: The 1- and 3-year survival of all patients was 73% and 47%. In the subgroup analyses, the combination of TACE and PEI (1) was associated with a longer survival (1-, 3-, 5-year survival: 90%, 52%, and 43%) compared to PEI treatment alone (2) (1-, 3-, 5-year survival: 65%, 50%, and 37%). Secondary PEI after initial stratification to TACE (3) yielded comparable results (1-, 3-, 5-year survival: 91%, 40%, and 30%) while PEI after stratification to best supportive care (4) was associated with decreased survival (1-, 3-, 5-year survival: 50%, 23%, 12%). Apart from the chosen treatment modalities, predictors for better survival were tumor number (n < 5), tumor size (< 5 cm), no ascites before PEI, and stable serum cholinesterase after PEI (P < 0.05). The mortality within 2 wk after PEI was 2.8% (n = 3). There were 24 (8.9%) major complications after PEI including segmental liver infarction, focal liver necrosis, and liver abscess. All complications could be managed non-surgically.
CONCLUSION: Repeated single-session PEI is effective in patients with advanced HCC at an acceptable and manageable complication rate. Patients stratified to a combination of TACE and PEI can expect longer survival than those stratified to repeated PEI alone. Furthermore, patients with large or multiple tumors in good clinical status may also profit from a combination of TACE and reconsideration for secondary PEI.
- Citation: Dettmer A, Kirchhoff TD, Gebel M, Zender L, Malek NP, Panning B, Chavan A, Rosenthal H, Kubicka S, Krusche S, Merkesdal S, Galanski M, Manns MP, Bleck JS. Combination of repeated single-session percutaneous ethanol injection and transarterial chemoembolisation compared to repeated single-session percutaneous ethanol injection in patients with non-resectable hepatocellular carcinoma. World J Gastroenterol 2006; 12(23): 3707-3715
- URL: https://www.wjgnet.com/1007-9327/full/v12/i23/3707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i23.3707
The prognosis of untreated HCC remains poor. The 3-year survival ranges from 21% to 28% and declines to about 8% for advanced tumors[1]. The only therapies that currently offer potential cure are surgical resection and liver transplantation. Despite improved surgical techniques, only about 20% of HCC are amenable to resection, mainly due to advanced tumor growth and the underlying cirrhosis[2]. Systemic chemotherapy has shown no significant survival benefit in comparison to best supportive care[3]. Hence, locoregional modalities like transarterial chemoembolisation (TACE), percutaneous ethanol injection (PEI), laser induced thermo therapy (LITT), and radiofrequency ablation (RFA) are employed widely in non-resectable HCC in an attempt to prolong survival[4-7]. TACE is the preferred modality for patients with large and disseminated HCC in good physical status, whereas repeated PEI has shown efficacy in cases of small tumors[8]. Both modalities have proven their effectiveness in terms of tumor response and survival, even more so when combined[9-17].
PEI has gained importance in small HCC due to its safety and ease of operation. The method is both effective and economically sound. The survival data of patients with small tumors after PEI are comparable to those after surgical resection with 5-year survival rates between 32%-59%[15,18-22]. The amount of ethanol applicable during one session was initially limited. With the introduction of the single-session high-dose technique under general anaesthesia by Livraghi and co-workers, the indications for PEI have been extended[23]. The injection of higher quantities of ethanol during one session under mechanical ventilation offers the advantage of treating larger tumors and more lesions at a time, requiring fewer interventions. Furthermore, tumors in difficult accessible areas (e.g. liver segments 8, 1 or 2) can be better accessed in deep inspiration under mechanical ventilation.
According to physical status and tumor extent patients with non-resectable HCC were stratified in an interdisciplinary conference to repeated single-session PEI, repeated TACE sessions, a combination of TACE and repeated single-session PEI or to best supportive care[24]. Aim of this prospective study was to evaluate all patients of our institution who received PEI treatment from 1999 to 2003. Using multivariate regression analyses of pre-treatment variables, survival predictors were identified. In subgroup analyses, patients stratified to repeated PEI alone were compared to those stratified to a combination of TACE and PEI. Furthermore, long-term results of those patients who were reevaluated and switched secondarily to PEI treatment after being initially stratified to repeated TACE and of those patients who received PEI after being initially stratified to best supportive care for advanced disease were analysed[9].
One hundred and one patients (age range 35-88 years, 85% male, mean observation time 26 ± 17 mo) with non-resectable histologically-confirmed HCC who received PEI treatment from July 1999 until July 2003 in our institution were included into the present study.
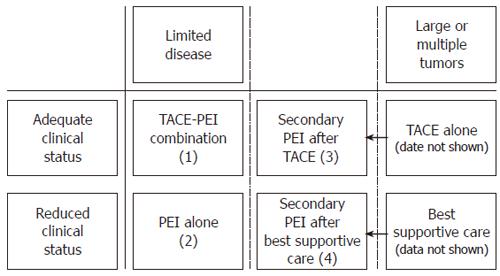
In an interdisciplinary gastroenterological-radiological conference, patients were stratified to one of the following treatment modalities according to physical status and tumor extent (Figure 1): combination of TACE and repeated single-session PEI, repeated single-session PEI alone, repeated TACE alone, or best supportive care. After thorough explanation of the procedures, informed consent was obtained from each patient prior to therapy. The data of the TACE-PEI combination group (1) and of the PEI alone (2) group were analyzed. In addition, patients with extensive disease (large or multiple tumors) were reconsidered and in some cases restratified to secondary PEI treatment (Figure 1). These patients were evaluated in two separate subgroups: patients switched secondarily to PEI after being initially stratified to repeated TACE (3) or after being initially stratified to best supportive care (4).
Contraindications against regional treatment were extrahepatic tumor manifestation, sepsis, hepatic encephalopathia, elevated serum ammonia > 47 μmol/L, platelet counts < 50 000/μL, prothrombin activity < 50%, serum creatinine >120 μmol/L, left ventricular ejection fraction < 50%, heart attack within the previous 12 mo, heart insufficiency >NYHA stage II. The inclusion into the liver transplantation program was not a contraindication.
Further contraindications against PEI were significant perihepatic ascites or inadequate parameters for general anaesthesia.
Further contraindications against TACE were portal vein thrombosis, impossible catheterization of the hepatic artery, cholinesterase (CHE) < 2 mU/mL (21°C), inadequate functional, hematological or biochemical parameters for chemotherapy.
Pre- and post-treatment evaluation included physical examinations, the assessment of clinical parameters, toxicities, adverse events and survival and the evaluation of tumor markers as well as hematological and biochemical parameters. To classify cardiac function and to detect right heart hypertrophism and pulmonary hypertension, echocardiography, cardiac scintigraphy, and ECG were performed.
Single-session PEI therapy was performed during general anaesthesia under realtime ultrasound guidance. Before each session the patients underwent a complete abdominal ultrasound examination. After thorough skin disinfection, absolute sterile ethanol (96%) was injected transcutaneously into the tumor using a 0.7 mm needle (Angiomed, Karlsruhe, Germany) and a biopsy-transducer (3.5 MHz, Toshiba, Neuss, Germany). Starting at the most distal part of the tumor, ethanol was continously injected while carefully retracting the needle until the tumor echogenicity became homogenous. After complete ethanol administration, short-term follow-up ultrasound control examinations were performed. Intravenous infusions of antibiotics and of fluid replacement were given before and for two days after PEI.
Six weeks after therapy, doppler ultrasound control examinations were performed. In case of detectable remaining tumor vascularisation (indicating remaining vital tumor tissue), tumor growth or new lesions, further PEI treatment was initiated. In case of uncertain vascularisation echocontrast enhanced ultrasound and a biphasic CT or contrast-enhanced MRI scan were performed. In case of no detectable tumor vascularization, patients were followed up every three months.
TACE was performed under local anaesthesia. Before each TACE session, a biphasic CT scan of the abdomen was performed to show tumor extent, and to check for possible extrahepatic disease. After aortography and a selective mesentericoportography to verify vascular anatomy and the patency of the portal vein, a selective hepaticography was performed using a standard diagnostic catheter or, if necessary, a coaxial system. According to tumor vascularisation and distribution a mixture of cisplatin (50 mg/m²), doxorubicin (50 mg/m²), 450 to 900 mg degradable starch microspheres (Spherex, Pharmacia, Erlangen, Germany) and 5 to 30 mL iodized oil (Lipiodol© Ultra-Fluide, Guerbet, Sulzbach/Ts., Germany) were administered under fluoroscopic control. In case of stasis or reflux, the injection was stopped until the arterial flow resumed. After complete administration, a control hepaticography was performed to document the arterial perfusion and the reduction of tumor vascularisation. Intraarterial analgesia (Dolantin©, Pethidin, Aventis, Bad Soden/Ts., Germany) was sufficient to control local pain in all cases. Intravenous infusions of antibiotics and fluid replacement were given before therapy and for two days after TACE, accordingly.
Data analysis was performed employing the Statistical Package for the Social Sciences Version 12.0 for Windows (SPSS© Inc., Chicago IL, USA). For comparison of distributions the Wilcoxon-test was used, for metric variables the chi-square test and the Fisher’s exact test for nominal variables. Results were presented as mean ± standard deviation or frequencies [n (%)].
Overall survival of all patients and survival of the subgroups were determined by Kaplan-Meier analyses. Kaplan-Meier curves were statistically compared by the log-rank-test. In order to quantify the influence of the treatment strategies on the survival outcome, a multivariate logistic regression analysis (enter method) was carried out. The clinical parameters that differed between the four treatment groups at baseline (Okuda-classification, presence of portal vein thrombosis, presence of ascites, number of tumors, maximum tumor diameter, and CHE) were entered into the regression model as well as Child-Pugh stage, α-fetoprotein (AFP), fever, incidence of complications, and the type of treatment administered. The model rendered the relevance of the variables tested competitively (R-square-value) and identified significant predictors for survival.
Subsequently, Kaplan-Meier curves were computed for all patients stratified separately for every significant predictor of the regression analysis in order to demonstrate the influence of the predictors on survival probabilities.
A P-value < 0.05 was considered significant, and a P-value < 0.001 was considered highly significant.
The patient set included a high proportion of patients with advanced stages of liver cirrhosis and tumor extent. Forty-one percent of the 101 patients presented with cirrhosis of Child-Pugh stage B, 15% with stage C. Okuda-scores II and III were present in 62%. Portal vein thrombosis was found in 18% and ascites requiring intensified diuretic therapy in 42% of the patients. In only 14% uninodular tumors were detected, in 70% up to 5 tumor lesions and in 15% multiple tumors. The mean maximum tumor size was 53 (± 27) mm in diameter. High mean AFP levels of 1188 ng/mL (± 6802) were seen, and the mean CHE levels were reduced to 3.3 mU/mL (± 1.6). The hepatitis B status was positive in 27% of the patients, and hepatitis C status in 22%.
The baseline characteristics of the four subgroups differed due to the selection criteria employed. A comparison of the frequencies and mean values of clinical variables is shown in Table 1.
| PEI alone | Sec PEI after bestsupp. care | TACE-PEIcombinat. | Sec PEI, afterTACE | P-value | ||
| (2) n = 34 | (4) n = 20 | (1) n = 37 | (3) n = 10 | |||
| Child-Pugh | A | 14 (41%)1 | 7 (35%) | 18 (49%) | 6 (60%) | |
| B | 14 (41%) | 10 (50%) | 19 (51%) | 4 (40%) | ||
| C | 6 (18%) | 3 (15%) | 0 (0%) | 0 (0%) | ||
| Okuda | 1 | 12 (36%) | 2 (10%) | 16 (46%) | 6 (67%) | 0.05 |
| 2 | 20 (61%) | 14 (74%) | 16 (46%) | 3 (33%) | ||
| 3 | 1 (3%) | 3 (16%) | 3 (8%) | 0 (0%) | ||
| Gender | male | 27 (80%) | 19 (95%) | 33 (89%) | 7 (70%) | NS |
| Portal vein thromb | yes | 1 (3%) | 7 (35%) | 9 (26%) | 1 (10%) | 0.02 |
| Ascites | yes | 12 (35%) | 13 (65%) | 16 (44%) | 1 (10%) | 0.03 |
| No of tumors | 1 | 4 (12%) | 3 (15%) | 5 (13%) | 2 (20%) | 0 |
| 2 | 8 (23%) | 3 (15%) | 4 (11%) | 1 (10%) | ||
| 3 | 13 (38%) | 1 (5%) | 2 (6%) | 2 (20%) | ||
| 4 | 7 (21%) | 0 (0%) | 9 (24%) | 0 (0%) | ||
| 5 | 2 (6%) | 1 (5%) | 17 (46%) | 1 (10%) | ||
| multiple | 0 (0%) | 12 (60%) | 0 (0%) | 4 (40%) | ||
| Tumor size | mm | 41 (14)2 | 68 (39) | 52 (20) | 75 (32) | 0 |
| No of PEI | n | 2.3 (1.6) | 3 (2.8) | 2.6 (1.2) | 3.3 (2.3) | NS |
| Weight | kg | 75 (12) | 75 (10) | 77 (16) | 78 (18) | NS |
| AFP | ng/mL | 235 (515) | 5743 (15 910) | 257 (476) | 28 (44) | NS |
| CRP | mg/L | 65 (61) | 92 (68) | 97 (73) | 83 (58) | NS |
| Bilirubin | µmol/L | 35 (24) | 36 (36) | 23 (21) | 13 (7) | 0.008 |
| CHE | mU/mL | 2.8 (1.6) | 2.6 (1.4) | 3.7 (1.4) | 4.6 (1.1) | 0.005 |
| LDH | U/L | 226 (77) | 218 (41) | 218 (58) | 198 (27) | NS |
| Protein | g/L | 73 (7) | 74 (8) | 73 (6) | 71 (4) | NS |
| AST | U/L | 43 (45) | 39 (26) | 37 (41) | 15 (6) | 0.01 |
| ALT | U/L | 37 (34) | 33 (24) | 35 (31) | 14 (5) | NS |
| AP | U/L | 204 (98) | 254 (125) | 212 (108) | 320 (412) | NS |
| G-GT | U/L | 105 (75) | 107 (75) | 153 (172) | 123 (203) | NS |
| Leukocytes | 1000/μL | 5.6 (2.2) | 5.5 (1.4) | 6.0 (2.5) | 6.3 (1.8) | NS |
| QUICK | % | 75 (18) | 79 (13) | 85 (18) | 88 (21) | NS |
| Thrombocytes | 1000/μL | 120 (56) | 145 (76) | 124 (62) | 213 (115) | NS |
| Creatinine | µmol/L | 82 (38) | 106 (120) | 78 (35) | 72 (17) | NS |
Significant deviations of the subgroups were shown for the Okuda score, the frequencies of portal vein thrombosis and ascites, the number of tumor lesions, the maximum size of tumor diameter and the liver parameters CHE, bilirubin, and AST.
Overall, 268 PEI-interventions were performed in 101 patients (2.7 ± 1.7 sessions per patient). The mean injected ethanol volume was 42 mL (3-117) per session. The mean hospitalisation time was 6.8 ± 4.8 d. In 43 patients (40.5%) intensified diuretic therapy was needed to reduce pre-treatment ascites. According to the stratification algorithm 37 patients received combination treatment of TACE and repeated PEI (1) and 34 patients received PEI alone (2). Ten patients primarily stratified to repeated TACE treatment only, were switched after TACE discontinuation to secondary PEI treatment (3). Another 20 patients who were primarily stratified to best supportive care, were considered treatable by PEI and received subsequently repeated PEI sessions in order to decrease tumor load in individual therapy settings (4).
Reasons for discontinuation of TACE in the patients continued secondarily on repeated PEI sessions were adverse events (n = 6), efficacy failure/progressive disease (n = 3) or achievement of preconditions for PEI treatment due to tumor regression (n = 1). In those patients primarily stratified to best supportive care secondary PEI treatment was performed because of improvement of general health status (n = 9), of patient´s request (n = 7), of reduction of ascites (n = 2), or as a bridge to liver transplantation (n = 2).
Seven of the 101 patients received liver transplants during follow-up (7%). Two of these patients had been stratified to combination treatment of TACE and PEI (1), four patients to repeated PEI alone (2), and one patient secondarily to repeated PEI after primary stratification to TACE (3).
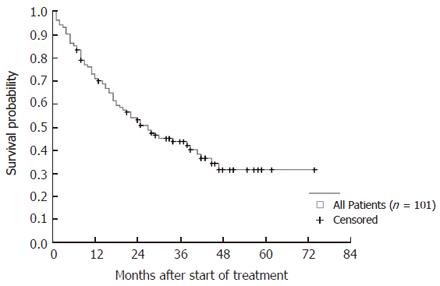
The overall 1- and 3-year-survival rates of the 101 patients were 73% and 47%, respectively. Kaplan-Meier analyses of all patients rendered survival probabilities of 72% after 1 year, 43% after 3 years, and 32% after 5 years (Figure 2).
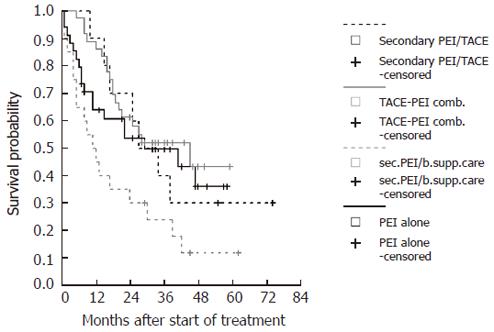
A comparison of the Kaplan-Meier survival curves for the four subgroups is shown in Figure 3. Survival probabilities differed between the subgroups. Significant differences were detected by log rank-tests comparing secondary PEI after best supportive care (4) to combination treatment of TACE and PEI (1) (P < 0.001) and to PEI treatment alone (2) (P < 0.05). There was a trend towards better survival in the combination group (1) compared to PEI alone (2) (P = 0.1).
The 1-, 3-, and 5-year survival probabilities of the subgroups were 90%, 52%, 43% for the combination group (1); 65%, 50%, 37% for the PEI alone group (2); 91%, 40%, 30% for the secondary PEI group after TACE discontinuation (3); and 50%, 23%, 12% for the secondary PEI group after best supportive care stratification (4).
From all clinical baseline characteristics that differed between the four subgroups at the outset of the study, pre-treatment ascites, the number of tumor lesions, the maximum tumor size, and CHE were significant predictors in the multivariate enter-model (Table 2). Additionally, the type of treatment administered proved to have independent predictive value in the model.
| Predictors | Cut-off | P-value | Odd's ratios | Confidenceinterval |
| AFP | < 400 ng/mL | NS | ||
| Fever | < 38°C | NS | ||
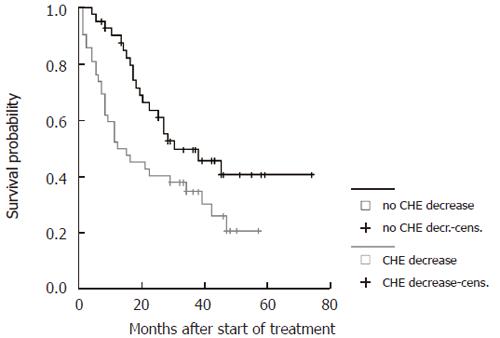
| CHE-level at follow up | Change < 1 mU/mL | 0.05 | 6 | 2.4-10.1 |
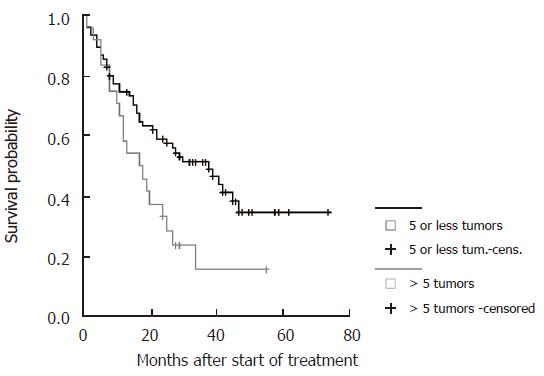
| No of tumor lesions | ≤ 5 | 0.01 | 11.1 | 3.3-22.3 |
| Child-Pugh | Child A | NS | ||
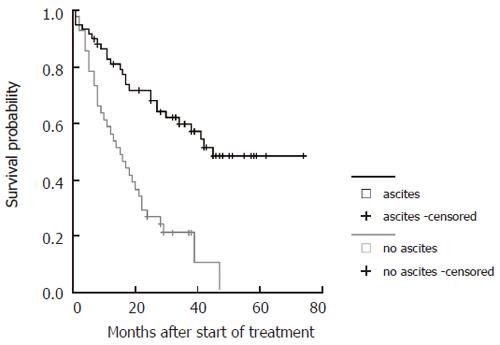
| Ascites | No ascites | 0.03 | 12.2 | 4.7-30.3 |
| Portal vein thrombosis | No thrombosis | NS | ||
| Okuda | Okuda stage 1 | NS | ||
| Severe complications | No complication | NS | ||
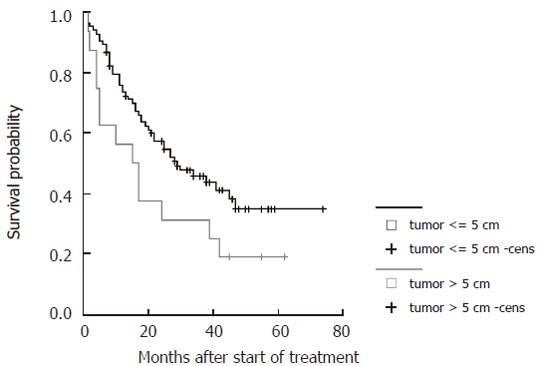
| Maximum tumor size | ≤ 5 cm | 0.04 | 6.1 | 1.3-17.5 |
| Type of treatment | TACE-PEI comb (I) vs other groups | 0.05 | 4.3 | 1.2-14.7 |
The probability to survive longer than three years was 6-fold higher in patients with stable CHE levels under therapy and in patients with tumors of less than 5 cm in maximum diameter, 11-fold higher in patients with five tumor lesions or less and 12-fold higher in patients without ascites prior to therapy. Treatment with TACE and PEI combination (1) led to a 4-fold higher survival probability compared to the other subgroups (2-4) independently of the clinical parameters tested in the model.
Survival curves were generated for all significant parameters of the prediction model and stratified according to the cut-offs employed (Figure 4, Figure 5, Figure 6 and Figure 7). The differences of the stratified subgroups were significant in all parameters shown (logrank-test: P < 0.05). The survival was improved significantly in patients with stable CHE-levels under therapy as a parameter of the liver function, in patients without ascites prior to therapy, in patients with a maximum tumor diameter of less than 5 cm, and in patients with five or less tumor lesions.
A total of 67 TACE procedures were performed. After 7 (10.4%) procedures complications were recorded. There were two cases of reversible leukopenia, one case of reversible pancytopenia, two cases of dissection of the hepatic artery, one case of reversible liver failure, and one inguinal hematoma.
Sixty-nine (25.7%) complications occurred after the 268 PEI sessions performed. Forty-five of these (16.8%) were considered minor [40 ascites (29 discrete, 7 intermediate, 4 severe), 3 pleural effusion, 1 oedema of the lower leg]. In 24 interventions (8.9%) major complications were observed (5 segmental liver infarctions, 2 focal liver necroses, 4 liver abscesses, 1 pulmonary embolism, 5 bleedings into liver or abdomen, 3 thromboses of the portal vein, 1 thrombosis of the superior mesenteric vein, 1 pancreatitis, 1 cholecystitis, and 1 cholangitis). All complications were managed non-surgically, and one liver abscess needed local drainage. There were no instances of needle-track seeding.
The mortality within 2 wk after PEI was 2.8% (3 patients). Two patients died with the signs of an acute right heart decompensation (one patient suffering from pre-existing alcoholic congestive heart disease with pulmonary hypertension, the other suffering from previously unknown right ventricular hypertrophy). The third patient developed a hypertensive crisis followed by an apoplectic insult.
In patients with HCC, survival is influenced by the etiology and characteristics of the tumors as well as the degree of cirrhosis and the remaining hepatic function. Since the degree of cirrhosis is rarely affectable in most cases, improval of survival can be expected mainly through effective tumor treatment. To date, there is still no standard treatment for advanced, unresectable HCC. The lack of efficient systemic chemotherapy against HCC and the non-resectability in most cases led to the development of various locoregional treatment modalities[22]. Increased anti-tumor efficacy might be achieved through a combination of different modalities carefully tailored to each patient.
PEI was introduced in 1983 and has become a standard treatment for small HCC[25]. The rationale for PEI is complete tumor necrosis resulting from cellular dehydration, protein denaturation, and chemical occlusion of tumor-feeding vessels. The method is best applicable in those HCCs that are surrounded by firm liver cirrhosis reducing the washout of ethanol. Ultrasound guidance allows for realtime monitoring of ethanol injection and distribution. Although regular PEI can be performed under local anaesthesia, general anaesthesia and endotracheal intubation have greatly facilitated the implementation of the single-session technique in terms of reduced pain and patient movement, thus extending the range of treatable tumors[23]. The major draw-back of PEI is a comparatively high recurrence rate of more than 30%[14]. Plausible reasons are inhomogeneous ethanol distribution within the tumor and the limited effect on extracapsular tumor spread. About 30% of small HCC (< 3 cm) have already developed invasive growth leading to microscopic intrahepatic metastases not detectable by current imaging[12]. The effect of PEI may be incomplete even in small tumors[26].
It is postulated that while the visible tumors are ablated by PEI, microscopic metastases are effectively destroyed by TACE. Furthermore, TACE is supposed to break up intratumoral septae and to create a fibrous wall around the hypervascularized tumors thus leading to a more homogenous distribution of ethanol during subsequent PEI[27,28]. Hence, the combination of TACE and repeated PEI, introduced in 1991, has shown superior results compared to PEI or TACE alone[11-14,29]. This concept was also adopted by our institution as described previously[24]. After careful interdisciplinary evaluation patients with non-resectable HCC were treated by a combination of TACE and repeated PEI, if possible. Those patients not amenable to combination treatment were stratified to either modality alone or to best supportive care according to their individual situation. Furthermore, patients with large or multiple tumors were reevaluated under therapy and secondarily switched to PEI when considered possible (Figure 1).
In the present prospective study there was a compara-tively high proportion of patients with decreased liver synthesis Child-Pugh B (41%) and C (15%) and portal vein thromboses (18%). Only 14% of the patients had single tumors while 15% had multiple disease and the mean tumor size was 53 mm (Table 1). Because both liver function and tumor extent affected therapy decisions, the present study was not a controlled trial but a prospective therapy evaluation based on a stratification scheme according to individual patient characteristics. The results were therefore not unexpected: patients stratified to PEI-therapy alone had worse pre-treatment conditions in terms of clinical status and liver function compared to those stratified to the combination of TACE and PEI (Table 1).
Overall observed 1- and 3-year-survival of all 101 patients was 72% and 47%. Kaplan-Meier analyses revealed a 1, 3, and 5 year survival probability of 90%, 52%, and 43% after initial stratification to TACE followed by PEI and of 65%, 50%, and 37% after PEI alone (Figure 3). Superior survival of combining TACE and PEI compared to either modality alone has been proven by several investigators. Bartolozzi et al detected superior recurrence-free survival of patients after combination of TACE and PEI (100% and 72% after 1 and 3 years), compared to repeated TACE alone[13]. Allgaier et al found a survival benefit of patients stratified to combination of TACE and PEI (vs PEI alone) due to a lower recurrence rate [11]. Koda et al showed superior survival after 1, 3, and 5 years (100%, 80%, and 40%) for patients after combination treatment (vs PEI alone), and Kamada et al recorded 1-, 3-, and 5-year survival rates of 90%, 65%, and 50% after combination treatment[12,14].
Our results are hence in line with the above data; however, there are differences in terms of patient criteria. The former studies did apply comparatively strict inclusion criteria in terms of tumor number and size. It should be noted that the patients consecutively included into the present study were in more advanced stages of both tumor extent and liver cirrhosis. Being a liver transplantation center in Northern Germany, advanced stages of tumor extent and cirrhosis were included in an attempt to prolong survival of those patients awaiting possible liver transplantation: patients with multinodular disease up to 5 tumors with less than 5 cm diameter or singular tumors up to 7 cm in diameter were primarily stratified to combination treatment. In this setting, the present survival data were higher than expected. The overall survival of our patients after combination therapy is comparable to reported survival data of tumor-free patients with liver cirrhosis[30]. Consequently, the present combination treatment of HCC can be considered effective for our patients with advanced disease.
The 10 patients with large (>7 cm) or multiple (n > 5) tumors who were switched from repeated TACE treatment to secondary PEI after re-evaluation (Figure 1), were indeed amenable to secondary PEI after receiving initial TACE as stated by other investigators[31]. Interestingly, 1-, 3-, and 5-year survival of this group (91%, 40%, and 30%) was comparable to those patients with limited disease who were primarily stratified to combination treatment (90%, 52%, and 43%) (Figure 3). From our experience it may be advisable to reconsider patients for PEI even after repeated TACE treatment, especially if TACE therapy cannot be continued.
The 1-, 3-, and 5-year-survival of the 20 patients with severely impaired liver function and advanced tumors, who were switched to repeated PEI after initially being stratified to best supportive care, was comparatively poor (50%, 23%, and 12%). However, it was superior to a large set of patients treated by best supportive care only or systemic therapy from our institution (1- and 3-year-survival: 32% and 2%)[24]. Large tumors can technically be treated by PEI as a salvage therapy, however, at the increasing risk of systemic, toxic side-effects and other severe complications as previously suggested[9]. From our experience we would not generally recommend single-session PEI as a salvage therapy in patients with both disseminated disease and severely impaired liver function who cannot receive TACE as initial treatment. Still, there may be patients in this group who might profit. It seems as if sufficient clinical status and/or the effect of initial TACE before PEI is needed to enhance anti-tumoral activity and survival.
In the multivariate analyses the parameters of limited tumor size and number, no ascites prior to therapy, and stable levels of CHE under therapy were associated with better survival. The type of treatment administered seemed to have independent predictive value, however, the significance of this result may be reduced due to the limited patient numbers (Table 2). Although screening programs improved the early detection of HCC, large non-resectable tumors are still frequently encountered and remain a major therapeutic challenge[32]. As expected, tumor size did affect survival. This might be explained by the fact that small tumors tend to have lower gradings than large tumors. Histological studies revealed that tumors up to 3 cm diameter are mostly well differentiated while large tumors are in general less differentiated[18,33,34]. Furthermore, there is a greater risk in larger HCC of peripheral satellite tumors that escape pre-treatment staging[35]. The functional status of the liver has been known to be a major predictor in PEI treatment as it is reflected in the significance of pre-treatment ascites and CHE-levels under therapy in the present study[19]. Both TACE and PEI might further impair the remaining liver function. The regeneration of functional liver parenchyma is possibly delayed as indicated by decreased liver synthesis parameters after treatment (data not shown).
While regular PEI is considered a low-risk procedure, severe complications, including death have been reported[36-38]. In the present study on repeated single-session PEI in advanced tumors the overall complication rate was 25.7% (n = 69) including 8.9% (n = 24) major complications, however, all could be managed non-surgically. We consider these instances acceptable because single session PEI represents a more aggressive approach than regular PEI[37]. Giorgio et al stated a mortality of 1.8% after single-session PEI[31]. There were also three deaths (2.8%) within 14 days after PEI among our patients. Two patients died with the signs of right ventricular decompensation, one patient with a hypertensive crisis followed by an apoplectic insult. Although there was no direct link to PEI, a connection seems possible, as other authors stated cases of pulmonary hypertension after PEI were possibly due to shunting[23]. We did not observe any instance of needle track seeding, as described by others[21].
In conclusion, in our set of patients with advanced non-resectable HCC the combination of TACE and repeated single-session PEI is effective and shows superior results compared to repeated single-session PEI alone. Even for patients with large (>7 cm) or multiple (n > 5) tumors initially stratified to repeated TACE treatment alone PEI treatment is effective in terms of survival at an acceptable complication rate. The predictors including limited tumor size and number, no pre-treatment ascites, and stable levels of CHE under therapy are independently associated with better survival. Our experience further suggests that in advanced disease carefully tailored combination therapy is more advantageous than single therapy. The optimal strategy against advanced HCC, however, has still to be determined by future trials.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
| 1. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 839] [Cited by in F6Publishing: 876] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 2. | Sturm JW, Keese MA, Bönninghoff RG, Wüstner M, Post S. [Locally ablative therapies of hepatocellular carcinoma]. Onkologie. 2001;24 Suppl 5:35-45. [PubMed] [Cited in This Article: ] |
| 3. | Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8:117-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Head HW, Dodd GD 3rd. Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127:S167-S178. [PubMed] [Cited in This Article: ] |
| 5. | Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159-S166. [PubMed] [Cited in This Article: ] |
| 6. | Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892-2899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Miyauchi Y, Himoto T, Kimura Y, Nakai S, Deguchi A, Yoneyama H. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:1426-1432. [PubMed] [Cited in This Article: ] |
| 8. | Acunaş B, Rozanes I. Hepatocellular carcinoma: treatment with transcatheter arterial chemoembolization. Eur J Radiol. 1999;32:86-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Survival benefit of cirrhotic patients with hepatocellular carcinoma treated by percutaneous ethanol injection as a salvage therapy. Scand J Gastroenterol. 2002;37:350-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kirchhoff T, Chavan A, Galanski M. Transarterial chemoembolization and percutaneous ethanol injection therapy in patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1998;10:907-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Allgaier HP, Deibert P, Olschewski M, Spamer C, Blum U, Gerok W, Blum HE. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanol injection--a single-center analysis including 132 patients. Int J Cancer. 1998;79:601-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 12. | Kamada K, Kitamoto M, Aikata H, Kawakami Y, Kono H, Imamura M, Nakanishi T, Chayama K. Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, Carrai M, Maltinti G, Capria A, Conte PF. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812-818. [PubMed] [Cited in This Article: ] |
| 14. | Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 15. | Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108. [PubMed] [Cited in This Article: ] |
| 16. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2502] [Cited by in F6Publishing: 2507] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 17. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1904] [Cited by in F6Publishing: 1912] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 18. | Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, Ueno H, Kosuge T. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001;34:707-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Pompili M, Rapaccini GL, Covino M, Pignataro G, Caturelli E, Siena DA, Villani MR, Cedrone A, Gasbarrini G. Prognostic factors for survival in patients with compensated cirrhosis and small hepatocellular carcinoma after percutaneous ethanol injection therapy. Cancer. 2001;92:126-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 20. | Ueno S, Tanabe G, Nuruki K, Oketani M, Komorizono Y, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Prognosis of hepatocellular carcinoma associated with Child class B and C cirrhosis in relation to treatment: a multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg. 2002;9:469-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62 Suppl 1:64-68. [PubMed] [Cited in This Article: ] |
| 23. | Livraghi T, Benedini V, Lazzaroni S, Meloni F, Torzilli G, Vettori C. Long term results of single session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer. 1998;83:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 24. | Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, Klempnauer J, Galanski M, Manns MP. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862-1868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Ebara M, Ohto M, Sugiura N, Kita K, Yoshikawa M, Okuda K, Kondo F, Kondo Y. Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol. 1990;5:616-626. [PubMed] [Cited in This Article: ] |
| 26. | Castroagudin JF, Delgado M, Martinez SM, Abdulkader I, Potel J, Tome S, Otero E, Varo E. Prospective histopathological analysis of hepatocellular carcinoma treated with percutaneous ethanol injection in patients on the waiting list for liver transplantation. Transplant Proc. 2005;37:1477-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259-2267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 28. | Dimitrakopoulou-Strauss A, Strauss LG, Gutzler F, Irngartinger G, Kontaxakis G, Kim DK, Oberdorfer F, van Kaick G. Pharmacokinetic imaging of 11C ethanol with PET in eight patients with hepatocellular carcinomas who were scheduled for treatment with percutaneous ethanol injection. Radiology. 1999;211:681-686. [PubMed] [Cited in This Article: ] |
| 29. | Tanaka K, Okazaki H, Nakamura S, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179:713-717. [PubMed] [Cited in This Article: ] |
| 30. | Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45-53. [PubMed] [Cited in This Article: ] |
| 31. | Giorgio A, Tarantino L, de Stefano G, Perrotta A, Aloisio V, del Viscovo L, Alaia A, Lettieri G. Ultrasound-guided percutaneous ethanol injection under general anesthesia for the treatment of hepatocellular carcinoma on cirrhosis: long-term results in 268 patients. Eur J Ultrasound. 2000;12:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 447] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 33. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 723] [Cited by in F6Publishing: 686] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 34. | Nanashima A, Tanaka K, Yamaguchi H, Shibasaki S, Morino S, Yoshinaga M, Sawai T, Nakagoe T, Ayabe H. Fibrosis and inflammatory activity in noncancerous tissue and mitotic index of cancer tissue in patients with hepatocellular carcinoma: relationship to clinicopathological factors and prognosis after hepatic resection. Dig Dis Sci. 2003;48:1517-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Vilana R, Bruix J, Bru C, Ayuso C, Solé M, Rodés J. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992;16:353-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 198] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Tapani E, Soiva M, Lavonen J, Ristkari S, Vehmas T. Complications following high-dose percutaneous ethanol injection into hepatic tumors. Acta Radiol. 1996;37:655-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Meloni F, Lazzaroni S, Livraghi T. Percutaneous ethanol injection: single session treatment. Eur J Ultrasound. 2001;13:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablation of hepatocellular carcinoma: state-of-the-art. Liver Transpl. 2004;10:S91-S97. [PubMed] [Cited in This Article: ] |