Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 14, 2006; 12(10): 1569-1576
Published online Mar 14, 2006. doi: 10.3748/wjg.v12.i10.1569
Published online Mar 14, 2006. doi: 10.3748/wjg.v12.i10.1569
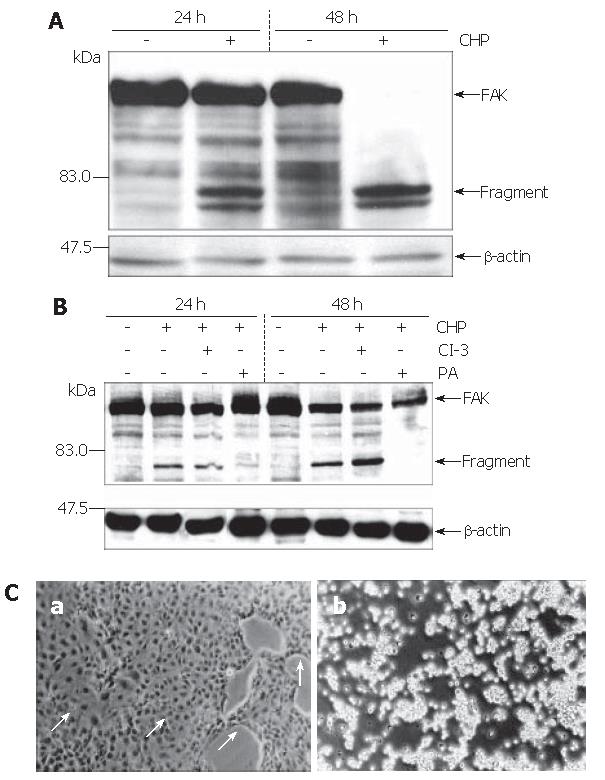
Figure 3 FAK fragmentations in DSL6A cells induced by CHP.
A Immunoblotting of protein extracted from DSL6A cells after being incubated for 16 h and 48 h with or without 10 mmol/L CHP. CHP induced a time-dependent degradation of the 125 ku FAK protein, resulting in fragments of about 80 ku. B Simultaneous addition of the Protease Arrest Reagent (PI) to the CHP-treated cells attenuated fragmentation of FAK; in contrast, the caspase-3 inhibitor z-DEVD-fmk (CI-3, 100 μmol/L) was not able to abolish the CHP-induced FAK degradation. To show equal protein loading, the membranes were stripped and re-probed using an anti-β-actin specific antibody. C Effect of CHP on morphological appearance of DSL6A cells; (a) simultaneous addition of 10 mmol/L CHP and a broad spectrum protease inhibitor (PI, Protease Arrest Reagent) abolished the CHP-induced loss of adherence; (b) In contrast to the broad spectrum protease inhibitor, the caspase-3 inhibitor z-DEVD-fmk (CI-3, 100 μmol/L) had no effect on the CHP-induced loss of adherence (Phase contrast microscopy, original magnification x 200).
- Citation: Mueller C, Emmrich J, Jaster R, Braun D, Liebe S, Sparmann G. Cis-hydroxyproline-induced inhibition of pancreatic cancer cell growth is mediated by endoplasmic reticulum stress. World J Gastroenterol 2006; 12(10): 1569-1576
- URL: https://www.wjgnet.com/1007-9327/full/v12/i10/1569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i10.1569