Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 28, 2005; 11(44): 7001-7006
Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.7001
Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.7001
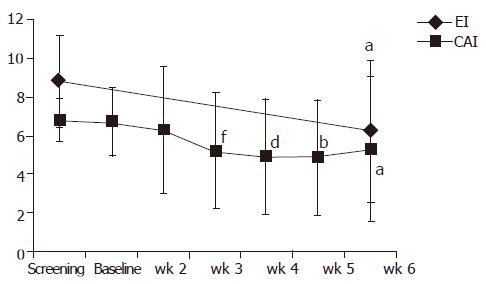
Figure 3 Course of clinical and endoscopic activity indices (CAI, EI) throughout the apheresis treatment.
aP<0.05 vs Et at wk 6, bP<0.01 vs Et at wk 5, dP<0.01 vs Et at wk 4, fP<0.05 vs Et at wk 3.
- Citation: Kruis W, Dignass A, Steinhagen-Thiessen E, Morgenstern J, Mössner J, Schreiber S, Vecchi M, Malesci A, Reinshagen M, Löfberg R. Open label trial of granulocyte apheresis suggests therapeutic efficacy in chronically active steroid refractory ulcerative colitis. World J Gastroenterol 2005; 11(44): 7001-7006
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/7001.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.7001