Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Jan 28, 2005; 11(4): 503-507
Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.503
Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.503
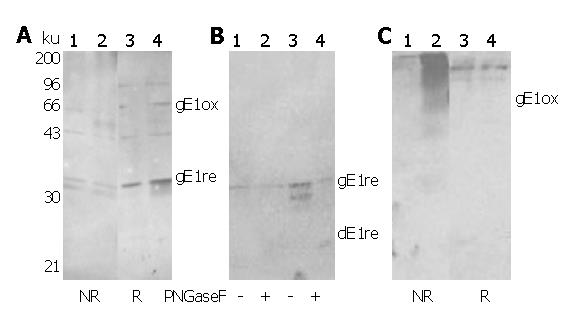
Figure 4 Detection of mammalian E1 glycoproteins using rabbit antisera against E.
coli-derived E1 proteins E1 glycoproteins expressed using recombinant vaccinia virus system were analyzed on standard SDS-PAGE followed by Western blot using RE1Z262W326R (A&B) and RE1Z262W326N (C) as primary antibody. In (A) and (C), lanes 1 and 3: HeLa cells infected with vvT7 alone; lanes 2 and 4: HeLa cells co-infected with vvT7 and vCEH-2. NR and R indicate non-reductive and reductive sample preparation, respectively. B: deglycosylation analysis of E1 glycoproteins using PNGase F. Lanes 1 and 2: HeLa cells infected with vvT7 alone; lanes 3 and 4: HeLa cells co-infected with vvT7 and vCEH-2. Positions of recognized E1 bands are indicated. Prefixed ‘g’ or ‘d’ indicates ‘glycosylated’ or ‘deglycosylated’ E1, respectively. Subscript ‘ox’ or ‘re’ indicates ‘oxidized’ or ‘reduced’ forms of E1, respectively.
-
Citation: Liu J, Zhu LX, Kong YY, Li GD, Wang Y. Purification and application of C-terminally truncated hepatitis C virus E1 proteins expressed in
Escherichia coli . World J Gastroenterol 2005; 11(4): 503-507 - URL: https://www.wjgnet.com/1007-9327/full/v11/i4/503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.503