Published online Oct 7, 2005. doi: 10.3748/wjg.v11.i37.5821
Revised: December 23, 2004
Accepted: December 26, 2004
Published online: October 7, 2005
AIM: The permissible exposure limit (PEL) of vinyl chloride monomer (VCM) in developed country was 1 p/m (2.79 mg/m3); and threshold limit value-short term exposure limit (TLV-STEL) in China was 11 times higher [11 p/m (30 mg/m3)] than it, till 2002. The mechanism of vinyl chloride monomer (VCM)-related carcinogenesis remains unclear. We aimed to analyze occupational health hazards exposure to doses lower than the Chinese occupational health standard in a selected VC polymerization plant in China, and also to elucidate the relationship between genetic polymorphisms and genetic susceptibility on liver lesions of workers exposed to VCM.
METHODS: In order to explore the mechanism of VCM-related health effects, we used a case-control design to investigate the association between the genetic polymorphisms of metabolic enzymes and liver lesions in workers occupationally exposed to VCM. Genotypes of CYP2E1, GSTT1, GSTM1, ALDH2 and ADH2 were identified using PCR and PCR-RFLP.
RESULTS: Even when the concentration of VCM was lower than the current Chinese occupational health standard, neurasthenia, pharyngeal irritation, liver ultrasonography abnormalities and hemoglobin disorders were significantly higher in exposure subjects compared to non-exposure subjects, and the relative risks (RR and 95% CI) were 1.74 (1.06-2.85), 1.97 (1.56-2.48), 10.69 (4.38-26.12), and 2.07 (1.20-3.57). CYP2E1 c1c2/c2c2 genotype was significantly associated with liver damages (OR 3.29, 95% CI 1.51-7.20, P < 0.01).
CONCLUSION: The incidences of neurasthenia and liver ultrasonography abnormalities significantly increase when the cumulative exposure dose increases. The genotypes of metabolic enzymes (CYP2E1 c1c2/c2c2, null GSTT1 and ADH2 1-1) play important roles in VCM metabolism. Polymorphisms of CYP 2E1, GSTT1 and ADH2 may be a major reason of genetic susceptibility in VCM-induced hepatic damage.
- Citation: Zhu SM, Ren XF, Wan JX, Xia ZL. Evaluation in vinyl chloride monomer (VCM)-exposed workers and the relationship between liver lesions and gene polymorphisms of metabolic enzymes. World J Gastroenterol 2005; 11(37): 5821-5827
- URL: https://www.wjgnet.com/1007-9327/full/v11/i37/5821.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i37.5821
Vinyl chloride monomer (CH2 = CHCl, VCM) is a colorless gas and a synthetic chemical that does not naturally occur. It is also known as monochloroethylene and chloroethene. At normal temperature it has a mild, sweet odor. Because VCM is easily polymerized to polyvinyl chloride, it is widely used in industry primarily. In fact, in the seventies of last century, 95% of the vinyl chloride produced was polymerized, and the rest is used in the formation of other chemicals. VCM was found to be easily absorbed through the respiratory tract, where it passed into the blood. Most studies concluded that the target organs for the VCM action are the liver, brain, lung, lymphohematopoietic system and skin, and hepatic lesion is its characteristic manifestation[1-3]. In 1974, the US National Institute of Occupational Safety and Health and US Centers for Disease Control and Prevention (CDC), reported that several deaths at one of their plants in a chemical company could be related to occupational exposures of vinyl chloride[4,5]. VCM has been classified by the International Agency for Research on Cancer (IARC) as a Group I carcinogen[6]. Since vinyl chloride is a known carcinogen, it has caused great concern to people who may be exposed to it.
PVC manufacture is a major industry in China, with total annual production of two or three million tons till now. In 1970’s, China began to produce PVC, the output increased from 780 000 tons in 1990 to nearly 2 400 000 tons in 2000, and the figure will increase to 3 million tons in the near future. Thousands of workers could be exposed to VCM. The permissible exposure limit (PEL) of VCM in developed country was 2.79 mg/m3, and in China which was 11 times higher (30 mg/m3) than it till 2002. Some studies of health hazards of workers exposure to VCM in China had been done. The results of these studies showed a high prevalence of abnormal liver function and neurasthenia as well as other symptoms due to exposure to VCM[7,8], and more tumors in VCM-exposed workers can be expected in future. However, the workers studied in the aforementioned studies were exposed to VCM at levels higher than the Chinese PEL. There is a general lack of information on estimates of health effects of workers exposed to lower levels of VCM exposure.
Earlier studies were more concerned with exposure levels and effects of vinyl chloride. More recently, there have been studies done on how vinyl chloride reacts in the body leading to cancer, both how it is bioactivated and how it interacts with DNA and forms DNA adducts. An earlier animal study has reported that VCM is primarily metabolized in the liver by CYP2E1 into active chloroethylene oxide (CEO), some of the CEO reacts to form adducts, 7-(2-oxoethyl) guanine. This adduct has been found in the most abundance, but it has not shown mispairing during DNA replication. Since CEO is unstable, it rearranges to chloroacetaldehyde (CAA)[9]. Once CAA is formed, it reacts with adenine, cytosine and guanine to form adducts. Reactions with guanine are less frequent than adenine and cytosine. There are four known etheno-adducts: 1, N6-ethenoadenine (eA); 3, N4 -ethenocytosine (eC); N2, 3-ethenoguanine (N2, 3-eG); and 1, N2 ethenoguanine (1, N2 eG)[10]. These adducts contain an additional five-membered ring system. Although these adducts are known to be formed, it is still unclear which one induces errors during DNA and RNA synthesis, for both of which may be reactive with DNA to form DNA adducts[11], and are mutagenic in bacterial systems and mammalian cells[12].
Most of population-attributable cancer heritability is related not to the rare deleterious gene defects but to polymorphic variations in the DNA sequence. CYP2E1 has already been reported to be associated with lung cancer in smokers[13]. In a recent study performed in the Caucasian population, CYP2E1 can account for only a small proportion of the variability in mutagenic response to VCM exposure[14]. Furthermore, CEO may then be metabolized by GSTs, and simultaneously CAA may be metabolized by ALDH2 and ADH2. Thus, those with appropriate types of ALDH2, ADH2, and GSTs may also have elevated reactive intermediates, which can lead to increased liver damages. The metabolism of VCM is mostly concerned with the enzymes CYP2E1,GSTT1, GSTM1, ALDH2 and ADH2. Several studies have shown that all the enzymes exhibit their genetic polymorphisms[15-17], thereby causing different biological effects of metabolizing enzymes.
We aimed to analyze occupational health hazards exposure to doses lower than the Chinese occupational health standard in a selected VC polymerization plant in China, and provide information whether occupational health standard of VCM exposure needs to be revised in China, and also to elucidate the relationship between genetic polymorphisms and genetic susceptibility on liver lesions of workers exposed to VCM.
Information was collected from workers at the VC polymeri-zation plant by using interviewer-administered questionnaires, subsequent to informed consent having been obtained during the medical surveillance process. The structured questionnaire contained questions that covered demographic characteristics, lifestyles, including cigarette-smoking habits and alcohol consumption, as well as compiling a detailed occupational history. Individuals who smoke once a day for over 6 mo were defined as smokers, and individuals who consumed once or more alcohol drinks a week for over 6 mo were considered drinkers.
Study subjects exposed to VCM for a period of >1 year in the plant were selected if the following criteria were met: detailed questionnaires had been completed; the hepatitis B, C viruses’ status were known; and a blood sample could be provided. At final tally, a total of 163 male and 75 female workers in the industry, with an average age of 33 years (range, 19-54 years) and an average exposure time of 7 years, and who had been occupationally exposed to VCM were included for analysis. One hundred and twenty four male and 88 female employees, with an average age of 38 years (range, 23-60 years), were selected as the non-exposure group, who worked in the same industry as the exposure subjects but not occupationally or environmentally exposed to VCM previously. The workers infected with hepatitis B, C viruses were excluded from the study because hepatitis B, C also leads to liver lesions.
Based on the results of medical examination, we divided the workers in the VCM exposure group into two groups. Fifty eight workers were selected as “liver lesion group” for their liver ultrasonography abnormality and/or alanine aminotransferase (ALT) >40 for at least 2 years and did not get acute hepatitis, chronic hepatitis and cholelithiasis. The “control subjects” were employees who worked at the same worksites as the liver damage group and also had exposure to VCM. Each control was matched for gender, duration of work, worksite, and cumulative exposure dose.
Exposure levels of VCM in the plant were based on existing environmental monitoring data. The plant kept VCM air concentration data, and the data were generated at different worksites since the beginning of its establishment. The level of VCM was calculated at different worksites of the company, which ranged from 0.3 p/m (0.85 mg/m3) to 17.8 p/m (48.41 mg/m3) in air, with a geometric average concentration was 2.6 p/m (7.11 mg/m3). And then the cumulative exposure dose of each worker was estimated relatively exact. The following equation was used to calculate cumulative exposure dose: Cumulative exposure dose (mg) = Σ (C * M * T) * A* 70%/106, where C (mg/m3): Geometric mean of VCM exposure concentration each year in a special workplace, calculated for all different worksites; M: number of exposure months of each year for a VCM worker; T: 2 h exposure time at each working day, 20 d in each month (exposure time per month was 2 400 min); and A: alveolar ventilation (male average: 6 500 mL/min, female average: 4 300 mL/min, 30% dead space).
Cumulative exposure dose in VCM exposure group ranged from 1 047.41 mg to 33 357.11 mg. Then the VCM exposure subjects were divided into high-exposure and low-exposure groups. The individuals placed into the high-exposure group had been exposed to a cumulative dose of >15 000 mg and those in the low-exposure group with their cumulative dose of ≤15 000 mg.
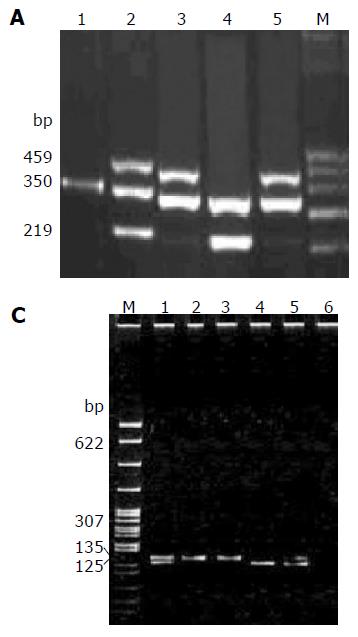
GSTT1 and GSTM1 genotypes were determined by co-amplification of two genes as previously described[18,19]. Amplification of human b-globin (350 bp) was also performed as a positive control in each reaction to confirm the presence of amplifiable DNA in the samples. The primers used for b-globin were 5’-GCC CTC TGC TAA CAA GTC CTA C-3’ and 5’-GCC CTA AAA AGA AAA TCG CCA ATC-3’. Individuals with one or more GSTT1 alleles had a 459-bp fragment and individuals with one or moreGSTM1 alleles had a 350-bp fragment (Figure 1A).
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was applied to detect polym-orphisms of the CYP2E1[20]. The primers used for CYP2E1 were 5’-TTC ATT CTG TCT TCT AAC TG-3’ and 5’-CAG TCG AGT CTA CAT TGT C -3’. The PCR products were digested with restricted endonuclease Pst I. Homozygous c1c1 individuals exhibited a product fragment of 410 bp, whereas homozygous c2c2 individuals revealed a 290-bp and a 120-bp fragment, and heterozygous c1c2 individuals demonstrated all the three fragments (Figure 1B).
The ALDH2-MboII polymorphism was determined by a modification of the methods developed by Harada and Zhang[21]. The sequences of ALDH2 primers were 5’-CAA ATT ACA GGG TCA ACT GCT ATG-3’ and 5’-CCA CAC TCA CAG TTT TCT CTT-3’. Homozygous 2-2 individuals demonstrated a single product fragment of 135 bp, whereas homozygous 1-1 individuals revealed both 125- and 10-bp fragments, and heterozygous 1-2 individuals exhibited all three of the fragments (Figure 1C).
According to Chen et al[22] the sequences of ADH2 primers were 5’- AAT CTT TTC TGA ATC TGA ACA G-3’and 5’- GAA GGG GGG TCA CCA GGT TGC-3’, and the PCR products were digested with Mae III. Homozygous 1-1 individuals demonstrated a single product fragment of 95 bp, whereas homozygous 2-2 individuals revealed both 60- and 35-bp fragments, and heterozygous 1-2 individuals exhibited all three of the fragments (Figure 1D).
SPSS 10.0 version and Epi-info 6.0 software packages were used for statistical analysis. Maentel-Haenszel c2-test was used to compare the differences between basic characteristics of the non-exposure and exposure group. Stratified analyses were used to explore the correlation between liver damages and genotypes of CYP2E1, GSTT1, GSTM1, ALDH2 and ADH2 among the workers exposed to different levels of VCM. Logistic regression model was used to select the best model and to estimate theOR of related risk factors. A P value less than 0.05 was considered statistically significant.
No significant difference was observed in age, duration of work, smoking, and drinking between the exposure and non-exposure subjects. All the standards of medical examination were selected as Chinese clinical diagnostic standards. There were significant differences in neurasthenia (P < 0.05), pharyngeal irritation (P < 0.01), liver ultrasonography abnormalities (P < 0.01) and hemoglobin disorders (P < 0.05) between the exposure and non-exposure groups, and relative risks were 1.74 (1.06-2.85), 1.97 (1.56-2.48), 10.69 (4.38-26.12), and 2.07 (1.20-3.57), respectively. Furthermore, abnormal ECG (P < 0.05, RR0.51, 95% CI 0.32-0.83) and fatty liver (P < 0.05, RR 0.42, 95% CI 0.20-0.88) were found significantly higher in non-exposure subjects as compared to exposure subjects. Other symptoms and signs did not show any statistical difference (Table 1).
| 0.42 (0.20-0.88)Symptoms and signs | Exposure group ) | Control group | Relative risk (RR |
| n = 238(%) | n = 212(%) | ||
| Neurastheniaa | 40 (16.81) | 21 (9.91) | 1.74 (1.06-2.58) |
| Pharyngeal irritationb | 138 (57.98) | 63 (29.72) | 1.97 (1.56-2.48) |
| Abnormal ECGa | 24 (10.08) | 40 (18.87) | 0.51 (0.32-0.83) |
| Cholelithiasis | 6 (2.52) | 7 (3.30) | 0.76 (0.26-2.24) |
| Renal cyst | 2 (0.84) | 3 (1.42) | 0.59 (0.10-3.52) |
| Liver ultrasonography abnormalityb | 51 (21.41) | 5 (2.36) | 10.69 (4.38-26.12) |
| Fatty livera | 10 (4.20) | 21 (9.91) | 0.42 (0.20-0.88) |
| Hemoglobin disordersa | 46 (19.33) | 22 (10.38) | 2.07 (1.20-3.57) |
| Hypertension | 11 (4.62) | 15 (7.07) | 0.64 (0.29-1.42) |
| Hepatic hemangioma | 3 (1.26) | 3 (1.42) | 0.89 (0.18-4.37) |
| Leucopenia | 4 (1.68) | 1 (0.47) | 3.61 (0.40-32.53) |
Trend chi-square test was used to compare symptoms and signs between the high-exposure and low-exposure groups. There were significant differences in neurasthenia, liver ultrasonography abnormality and pharyngitis between the high-exposure and low-exposure groups (P < 0.05), with the chi-square values of 6.41, 5.33 and 4.56, respectively. Their incidences increased when the cumulative exposure dose increased (Table 2). However, the other symptoms and signs did not show any statistical difference.
| Symptoms and signs | VCM exposure | |||||
| ≤ 15 000 mg | > 15 000 mg | Total | ||||
| (n = 186) | (n = 52) | (n = 238) | ||||
| Neurastheniaa | 26 | 14.00% | 14 | 26.92% | 40 | 16.81% |
| Respiratory system | 3 | 1.60% | 1 | 1.92% | 4 | 1.68% |
| Digestive system | 4 | 2.15% | 1 | 1.92% | 5 | 2.10% |
| Pharyngitisa | 104 | 55.90% | 34 | 65.38% | 138 | 57.98% |
| ECG | 19 | 10.20% | 5 | 9.61% | 24 | 10.08% |
| Nephridium USG | 1 | 0.53% | 2 | 3.85% | 3 | 1.26% |
| Gall bladder USG | 4 | 2.15% | 4 | 7.69% | 8 | 3.36% |
| Liver USGa | 34 | 18.30% | 17 | 32.69% | 51 | 21.43% |
| Fatty liver | 7 | 3.76% | 3 | 5.77% | 10 | 4.20% |
| Hepatic hemangioma | 1 | 0.53% | 2 | 3.85% | 3 | 1.26% |
| Splenomegaly | 8 | 4.30% | 2 | 3.85% | 10 | 7.08% |
Univariate analysis showed that CYP2E1 c1c2/c2c2 genotype was significantly associated with liver damages (OR 3.29, 95% CI 1.51-7.20, P < 0.01). Moreover, our results showed that the older or drinking subjects suffered from a higher frequency of liver damages but without statistical significance (OR 2.10, 95% CI 0.73-3.43, and OR 2.48, 95% CI 0.80-7.66). However, other factors did not show any association with liver damages (Table 3).
| VCM exposure group | Adjusted OR (95%CI) | ||
| Control(n = 58) | Liver lesions(n = 58) | ||
| GSTT1 | |||
| Non-null | 31 (53.4%) | 37 (63.8%) | 1.0 (reference) |
| Null 27 | (46.6%) | 21 (36.2%) | 0.65 (0.31-1.37) |
| GSTM1 | |||
| Non-null | 37 (63.8%) | 36 (62.1%) | 1.0 (reference) |
| Null | 21 (36.2%) | 22 (37.9%) | 1.08 (0.51-2.29) |
| CYP2E1 | |||
| c1c1 | 43 (74.1%) | 27 (46.6%) | 1.0 (reference) |
| c1c2/ c2c2 | 13/2 (25.8%) | 24/7 (53.4%) | 3.29 (1.51-7.20) b |
| ALDH2 | |||
| 1-1 | 37 (63.8%) | 36 (62.1%) | 1.0 (reference) |
| 1-2/2-2 | 17/4 (36.2%) | 17/5 (37.9%) | 1.08 (0.51-2.29) |
| ADH2 | |||
| 1-1 | 4 (6.9%) | 9 (15.5%) | 1.0 (reference) |
| 1-2/2-2 | 24/30 (93.1%) | 22/27 (84.5%) | 0.40 (0.12-1.39) |
| Age (yr) | |||
| < 35 | 41 (70.7%) | 35 (60.3%) | 1.0 (reference) |
| ≥ 35 | 17 (29.3%) | 23 (46.6) | 2.10 (0.76-3.43) |
| Drinking | |||
| No | 53 (91.4%) | 47 (81.0%) | 1.0 (reference) |
| Yes | 5 (9.6%) | 11 (19.0%) | 2.48 (0.80-7.66) |
| Smoking | |||
| No | 40 (69.0%) | 41 (71.7%) | 1.0 (reference) |
| Yes | 18 (31.0%) | 17 (29.3%) | 0.92 (0.42-2.04) |
ORs of liver lesions were calculated to investigate the joint effect of VCM exposure and genotypes. CYP2E1 c1c2/c2c2 genotype was significantly associated with liver lesions (OR 4.6, 95% CI 1.4-15.1, P < 0.05) in high-exposure group; and in low-exposure group, the distribution of non-null GSTT1 was higher in the control group compared to liver lesion group (OR 0.3, 95% CI 0.1-0.9, P < 0.05), and ADH2 1-2/2-2 genotype was the same (OR 0.2, 95% CI 0.1 -1.0, P < 0.05). The distribution of other genotypes did not have significant difference (Table 4).
| Genotypes | Cumulative VCM exposure | |||||
| ≤ 15 000 mg | > 15 000 mg | |||||
| Control | Liver lesion | OR (95% CI) | Control | Liver lesion | OR (95% CI) | |
| GSTT1 | ||||||
| Null | 15 | 23 | 1 | 16 | 14 | 1 |
| GSTM1 | 17 | 9 | 0.3 (0.1-0.9)a | 10 | 12 | 1.4 (0.5-4.1) |
| GSTM1 | ||||||
| Non-null | 21 | 21 | 1 | 16 | 15 | 1 |
| Null | 11 | 11 | 1.0 (0.4-2.8) | 10 | 11 | 1.2 (0.4-3.6) |
| CYP2E1 | ||||||
| c1c1 | 23 | 16 | 1 | 20 | 11 | 1 |
| c1c2/ c2c2 | 9 | 16 | 2.6 (0.9-7.2) | 6 | 15 | 4.6 (1.4-15.1)a |
| ALDH2 | ||||||
| 1-1 | 20 | 22 | 1 | 17 | 14 | 1 |
| 1-2/2-2 | 12 | 10 | 0.8 (0.3-2.1) | 9 | 12 | 1.6 (0.5-5.0) |
| ADH2 | ||||||
| 1-1 | 2 | 8 | 1 | 2 | 1 | 1 |
| 1-2/2-2 | 30 | 24 | 0.2 (0.1-1.0)a | 24 | 23 | 2.1 (0.2-24.5) |
Logistic regression analysis was employed to model the relationship between liver damages and genotypes of metabolic enzymes and related indicator among VCM workers. We observed a significant association between CYP2E1 c1c2/ c2c2 and liver lesions (P < 0.001, OR 3.173, 95%CI 1.329-7.572) and drinking (P = 0.10, OR 3.10, 95%CI 0.78-12.64), which might be associated with increased OR on liver lesions. But, we could not found any association between liver damages and age, duration of work, smoking and gender (Table 5).
| Logistic regression analysis | |||
| Coefficient | P | OR (95% CI) | |
| Age (yr) | 0.47 | 0.33 | 1.60 (0.62-4.19) |
| Duration of work | 0.24 | 0.60 | 1.27 (0.52-3.12) |
| Drinking | 1.14 | 0.10 | 3.14 (0.78-12.64) |
| Smoking | -0.9 | 0.13 | 0.41 (0.13-1.29) |
| Gender | -0.06 | 0.91 | 0.94 (0.36-2.47) |
| GSTT1 | -0.51 | 0.23 | 0.60 (0.26-1.39) |
| GSTM1 | -0.15 | 0.75 | 0.86 (0.36-2.10) |
| CYP2E1 | 1.16 | 0.009b | 3.17 (1.33-7.57) |
| ALDH2 | 0.09 | 0.84 | 1.09 (0.46-2.58) |
| ADH2 | -0.72 | 0.31 | 0.49 (0.12-1.96) |
| Constant | 0.24 | 0.84 | |
Several studies have proved VCM as a human carcinogen, and hence VCM exposure has strictly been regulated in many countries. In the USA, the Occupational Safety & Health Administration (OSHA) has adopted a permissible exposure limit (PEL) of 1 p/m for VCM as an 8 h time-weighed average (TWA). Taiwan has also adopted 1 p/m as the health standard. In many developing countries, for some reasons, they have not restricted the emissions of VCM to satisfy this standard. Therefore, their occupational health standard for VCM is still at a relatively higher level, for example, in China, the maximum allowable dose was 25 mg/m3 which was 9-fold higher than that in the advanced countries (Since June 2002 the TWA has been revised to 10 mg/m3 in China, and the maximum allowable dose modified to 25 mg/m3).
In this study, the level of VCM was lower than National Occupational Health Standard of China, but the incidences of neurasthenia, pharyngeal irritation and liver USG abnormality in the exposure group were still higher compared to non-exposure group, which indicated that even the concentration is around 9 p/m, we should also pay attention to the damage induced by VCM. By modeling the relationship between the symptoms and cumulative exposure dose, the results showed that when cumulative exposure dose increased, the incidence of the symptoms increased in accordance.
We found that, under the same working environment and exposure to the same level of VCM, not all but only some workers in VCM-exposure group had got liver damages, which indicated that there are genetic susceptible differences among the workers. Then we used case-control design to investigate the association between the gene polymorphisms of metabolic enzymes and liver lesions.
VCM is metabolized to CEO (chloroethylene oxide) and chloroacetaldehyde (CAA) by CYP2E1, which bind to macromolecular surface and form the electrophilic metabolites that can induce liver lesions, even worsen to hepatic angiosarcoma. A study showed the efficiency of conversion of c2 was higher than c1[23]. Studies by Huang et al[24] and Li et al[14] showed that the frequency of the CYP2E1 c2c2 genotype was higher in the case group compared to the control group, but the degree of CYP2E1 necessary to influence genetic susceptibility due to exposure to VCM was different between the Chinese and the Caucasian population. In our study, we found that the frequency of CYP2E1 c1c2/c2c2 was higher in liver lesions group than that in controls. Stratified by cumulative exposure dose, in high dose group, the CYP2E1 c1c2/ c2c2genotype had higher frequency in liver lesions group than that in controls, indicating that CYP2E1 c1c2/c2c2 genotype might be the main cause of genetic susceptibility of liver damage, which is consistent with previous reports.
All the metabolizing products are further metabolized and detoxified by GSTs; VCM metabolites can also directly bind to glutathione (GSH) after catalyzation by GSTs. This investigation showed that in liver lesions group, the frequency of the GSTT1-null genotype was 63.8%, which was higher than 47.4% as reported by Taiwan scholars[24]. And the frequency of theGSTM1-null genotype was 62.1%, which was consistent with 60.5% as reported by other scholars[23,24]. The study manifested that GSTT1 non-null genotype protected the workers from exposure to low level VCM, but another study indicated that GSTT1 non-null genotype could form reactive metabolites and produce adverse effects[25], which resulted in liver damages. However, we could not see any correlation between GSTM1 and liver damage.
CEO may spontaneously transform into CAA, which may be subsequently metabolized by ALDH2. Although individuals with variant ALDH2*2 allele have a lower enzyme activity, polymorphism of ALDH2 has been reported to be associated with alcoholism[26]. Previous studies manifested that CEO produced DNA alkylating agent but CAA could not, suggesting that ALDH2had little effect on liver damage[27]. Our study also supports these results.
We found that ADH2 genotypes ADH2 1-2/2-2 and 1-1 were significantly different between liver lesions and controls in low-exposure group, which might be because the meta-bolized efficiency of ADH2*2 was higher than ADH2*1 [28]. Moreover, the incidence of liver damage in ADH2 1-2/2-2 might be lower than 1-1, if they were exposed to the same concentration of VCM.
The workers in our control group might have some potential VCM exposure based on environmental monitoring in the factory. However, our multivariate adjustment corroborates the possible mechanisms of VCM metabolism and carcinogenesis. In conclusion, VCM workers exposed to high concentration of VCM and with CYP2E1 c1c2/c2c2 genotype may have a higher risk of liver lesions, and the incidence of liver damage in ADH2 1-2/2-2 maybe lower than 1-1, if they were exposed to the same concentration of VCM.
We thank Dr. Shang-Jian Chai and Yu-Fang Li for their help in medical examination and VCM exposure data collection. We are very grateful to Dr. Li Jin (Department of Environmental Health, University of Cincinnati, 3223 Eden Ave, Cincinnati Ohio 45267-0056) for his critical review and scientific editing.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
Co-first-authors: Shou-Min Zhu, Xue-Feng Ren
| 1. | Uzych L. Human male exposure to vinyl chloride and possible teratogenic and mutagenic risks: a review. Hum Toxicol. 1988;7:517-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Laplanche A, Clavel-Chapelon F, Contassot JC, Lanouzière C. Exposure to vinyl chloride monomer: results of a cohort study after a seven year follow up. The French VCM Group. Br J Ind Med. 1992;49:134-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | John Luo JC, Cheng TJ, Du CL, Wang JD. Molecular epidemiology of plasma oncoproteins in vinyl chloride monomer workers in Taiwan. Cancer Detect Prev. 2003;27:94-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Althouse R, Huff J, Tomatis L, Wllbourn J. An evaluation of chemicals and industrial processes associated with cancer in humans based on human and animal data: IARC Monographs Volumes 1 to 20. Cancer Res. 1980;40:1-12. [PubMed] [Cited in This Article: ] |
| 5. | Cheng TJ, Huang ML, You NC, Du CL, Chau TT. Abnormal liver function in workers exposed to low levels of ethylene dichloride and vinyl chloride monomer. J Occup Environ Med. 1999;41:1128-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Dietz A, Langbein G, Permanetter W. [Vinyl chloride induced hepatocellular carcinoma]. Klin Wochenschr. 1985;63:325-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Du CL, Wang JD. Increased morbidity odds ratio of primary liver cancer and cirrhosis of the liver among vinyl chloride monomer workers. Occup Environ Med. 1998;55:528-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Mastrangelo G, Fedeli U, Fadda E, Valentini F, Agnesi R, Magarotto G, Marchì T, Buda A, Pinzani M, Martines D. Increased risk of hepatocellular carcinoma and liver cirrhosis in vinyl chloride workers: synergistic effect of occupational exposure with alcohol intake. Environ Health Perspect. 2004;112:1188-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | el Ghissassi F, Barbin A, Bartsch H. Metabolic activation of vinyl chloride by rat liver microsomes: low-dose kinetics and involvement of cytochrome P450 2E1. Biochem Pharmacol. 1998;55:1445-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Dosanjh MK, Chenna A, Kim E, Fraenkel-Conrat H, Samson L, Singer B. All four known cyclic adducts formed in DNA by the vinyl chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc Natl Acad Sci USA. 1994;91:1024-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Guengerich FP. Roles of the vinyl chloride oxidation products 1-chlorooxirane and 2-chloroacetaldehyde in the in vitro formation of etheno adducts of nucleic acid bases [corrected]. Chem Res Toxicol. 1992;5:2-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Cheng KC, Preston BD, Cahill DS, Dosanjh MK, Singer B, Loeb LA. The vinyl chloride DNA derivative N2,3-ethenoguanine produces G----A transitions in Escherichia coli. Proc Natl Acad Sci USA. 1991;88:9974-9978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Wu X, Shi H, Jiang H, Kemp B, Hong WK, Delclos GL, Spitz MR. Associations between cytochrome P4502E1 genotype, mutagen sensitivity, cigarette smoking and susceptibility to lung cancer. Carcinogenesis. 1997;18:967-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Li Y, Marion MJ, Ho R, Cheng TJ, Coulibaly D, Rosal R, Brandt-Rauf PW. Polymorphisms for vinyl chloride metabolism in French vinyl chloride workers. Int J Occup Med Environ Health. 2003;16:55-59. [PubMed] [Cited in This Article: ] |
| 15. | Li Z, Tan W, Shao K. Susceptibility to lung cancer in Chinese is associated with genetic polymorphism in cytochrome P4502E1. Zhonghua ZhongLiu ZaZhi. 2000;22:5-7. [PubMed] [Cited in This Article: ] |
| 16. | Wall TL, Peterson CM, Peterson KP, Johnson ML, Thomasson HR, Cole M, Ehlers CL. Alcohol metabolism in Asian-American men with genetic polymorphisms of aldehyde dehydrogenase. Ann Intern Med. 1997;127:376-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wong NA, Rae F, Simpson KJ, Murray GD, Harrison DJ. Genetic polymorphisms of cytochrome p4502E1 and susceptibility to alcoholic liver disease and hepatocellular carcinoma in a white population: a study and literature review, including meta-analysis. Mol Pathol. 2000;53:88-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ambrosone CB, Sweeney C, Coles BF, Thompson PA, McClure GY, Korourian S, Fares MY, Stone A, Kadlubar FF, Hutchins LF. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 2001;61:7130-7135. [PubMed] [Cited in This Article: ] |
| 19. | Guo JY, Wan DS, Zeng RP, Zhang Q. The polymorphism of GSTM1, mutagen sensitivity in colon cancer and healthy control. Mutat Res. 1996;372:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Murata M, Watanabe M, Yamanaka M, Kubota Y, Ito H, Nagao M, Katoh T, Kamataki T, Kawamura J, Yatani R. Genetic polymorphisms in cytochrome P450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione S-transferase (GST) M1 and GSTT1 and susceptibility to prostate cancer in the Japanese population. Cancer Lett. 2001;165:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Harada S, Zhang S. New strategy for detection of ALDH2 mutant. Alcohol Alcohol Suppl. 1993;1A:11-13. [PubMed] [Cited in This Article: ] |
| 22. | Chen WJ, Loh EW, Hsu YP, Cheng AT. Alcohol dehydrogenase and aldehyde dehydrogenase genotypes and alcoholism among Taiwanese aborigines. Biol Psychiatry. 1997;41:703-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Wan J, Shi J, Hui L, Wu D, Jin X, Zhao N, Huang W, Xia Z, Hu G. Association of genetic polymorphisms in CYP2E1, MPO, NQO1, GSTM1, and GSTT1 genes with benzene poisoning. Environ Health Perspect. 2002;110:1213-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Huang CY, Huang KL, Cheng TJ, Wang JD, Hsieh LL. The GST T1 and CYP2E1 genotypes are possible factors causing vinyl chloride induced abnormal liver function. Arch Toxicol. 1997;71:482-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Eaton DL. Biotransformation enzyme polymorphism and pesticide susceptibility. Neurotoxicology. 2000;21:101-111. [PubMed] [Cited in This Article: ] |
| 26. | Chen WJ, Loh EW, Hsu YP, Chen CC, Yu JM, Cheng AT. Alcohol-metabolising genes and alcoholism among Taiwanese Han men: independent effect of ADH2, ADH3 and ALDH2. Br J Psychiatry. 1996;168:762-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Guengerich FP, Mason PS, Stott WT, Fox TR, Watanabe PG. Roles of 2-haloethylene oxides and 2-haloacetaldehydes derived from vinyl bromide and vinyl chloride in irreversible binding to protein and DNA. Cancer Res. 1981;41:4391-4398. [PubMed] [Cited in This Article: ] |
| 28. | Takeshita T, Yang X, Inoue Y, Sato S, Morimoto K. Relationship between alcohol drinking, ADH2 and ALDH2 genotypes, and risk for hepatocellular carcinoma in Japanese. Cancer Lett. 2000;149:69-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |