Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5433
Revised: September 6, 2004
Accepted: September 9, 2004
Published online: September 21, 2005
AIM: To determine the effect of allitridi on cell cycle of human gastric cancer (HGC) cell lines MGC803 and SGC7901 and its possible mechanism.
METHODS: Trypan blue dye exclusion was used to evaluate the proliferation, inhibition of cells and damages of these cells were detected with electron microscope. Flow cytometry and cell mitotic index were used to analyze the change of cell cycle, immunohistochemistry, and RT-PCR was used to examine expression of the p21WAF1 gene.
RESULTS: MGC803 cell growth was inhibited by allitridi with 24 h IC50 being 6.4 μg/mL. SGC7901 cell growth was also inhibited by allitridi with 24 h IC50 being 7.3 μg/mL. After being treated with allitridi at the concentration of 12 μg/mL for 24 h, cells were found to have direct cytotoxic effects, including broken cellular membrane, swollen and vesiculated mitochondria and rough endoplasmic reticula, and mass lipid droplet. When cells were treated with allitridi at the concentration of 3, 6, and 9 μg/mL for 24 h, the percentage of G0/G1 phase cells was decreased and that of G2/M phase cells was significantly increased (P = 0.002) compared with those in the group. When cells were treated with allitridi at the concentration of 6 μg/mL, cell mitotic index was much higher (P = 0.003) than that of control group, indicating that allitridi could cause gastric cancer cell arrest in M phase. Besides, the expression levels of p21WAF1 gene of MGC803 cells and p21WAF1 gene of SGC7901 cells were remarkably upregulated after treatment.
CONCLUSION: Allitridi can cause gastric cancer cell arrest in M phase, and this may be one of the mechanisms for inhibiting cell proliferation. Effect of allitridi on cells in M phase may be associated with the upregulation of p21WAF1 genes. This study provides experimental data for clinical use of allitridi in the treatment of gastric carcinoma.
- Citation: Ha MW, Ma R, Shun LP, Gong YH, Yuan Y. Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J Gastroenterol 2005; 11(35): 5433-5437
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5433
Garlic has been used for over 3 000 years in traditional Chinese medicine and is widely used in preventing manifold diseases all over the world. Garlic is the bulb of onion genus lily family plants. Allitridi is a compound extracted from the garlic bulb. It is an important constituent of garlic oil containing diallyl trisulfide (DATS) and diallyl disulfide (DADS)[1,2].
Epidemiological studies indicate that garlic has remarkable antithrombotic, hypolipidemic, hypocholesterolemic and antineoplastic effects. Allitridi has been shown to have anticancer activities, such as inhibiting nitrate reductase, enhancing anticancer activity of macrophages, increasing sensitivity of anticancer drugs[3]. However, its definitive mechanism of anticancer activities has not been established. In this study, human gastric cells MGC803 and SGC7901 were treated with allitridi to study its effect on cell cycle arrest of human gastric cancer (HGC) cell lines MGC803 and SGC7901 and its possible mechanism.
MGC803 was the human gastric mucinous adenocarcinoma cell line and SGC7901 was the human gastric carcinoma metastatic lymph node cell line. These cell lines were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 50 U/mL penicillin and 50 μg/mL streptomycin. The cells were maintained at 37 °C in a humidified atmosphere containing 50 mL/L CO2 and 95% air. Viability of the cells used in these experiments was consistently more than 95%, when evaluated by the trypan blue exclusion method.
The effect of allitridi on cell growth and viability was determined by counting the number of cells. The cells at a density of 2.5105/mL were seeded onto 24-well plates, and treated with allitridi with different concentrations for 24 h. Cells in control group were also incubated simultaneously. Cell proliferation and inhibition curves were drawn and drug IC50 was detected.
Cells (1×106) were collected after being induced by allitridi at 12 μg/mL for 24 h, washed twice with PBS, centrifuged for 5 min at 1 500 r/min, fixed with 2% glutaral at 4 °C and dessicated, embedded in EPON-812. They were then double stained with uranium and plumbum, and observed under transmission electron microscope.
Flow cytometry was employed to determine the DNA content of these cells. The cells were seeded onto 100-mm dishes and grown in RPMI-1640 supplemented with 10% FCS. After being treated with allitridi at the concentration of 3, 6, and 9 μg/mL for 24 h respectively, the cells were harvested. The cells were trypsinized and washed with D-PBS. The sample was fixed by slowly dropping 2 mL of cold 70% ethanol into the tube and stored at 4 °C. After fixation, the cells were washed, re-pelleted, and re-suspended in 0.05 mg/mL propidium iodide (Fluka Co.), 100 U/mL RNase (Fluka Co.) in PBS. The sample was incubated at room temperature for 30 min, and analyzed on a FACSCalibur (BD PharMingen). Cell cycle data originally obtained with CellQuest software (BD PharMingen) were re-analyzed using ModFit software (Verity Software House, Topsham, ME, USA). Next, 6 μg/mL allitridi was added to the cultured cells. The induced cells were collected after 24 h. At the same time the negative control was constructed. The cells were smeared and stained with Giemsa dye. The mitotic cells in 1 000 cells were counted under light microscope, and the mitotic index of the cells was calculated.
The p21 protein was detected by immunohistochemistry using specific mAb (Maxin Co.). After being treated with allitridi at concentrations of 3, 6, and 9 μg/mL for 24 h, the cells were harvested. At the same time, the negative contrast was constructed. The cells were made into smears and stained using normal S-P immunohistochemical method. Color was displayed with DAB reagent and stained again with hernatein. Results were observed under light microscope. The cells had brown nuclei and plasma was positive.
The expression of p21 and p16 mRNA was tested by RT-PCR. An aliquot containing 5 μg/mL of RNA was used for the first-strand cDNA synthesis (TaKaRa). In each reaction, 100 μL solution contained 3 μmol/L of random hexamers, 25 mmol/L Tris-HCl, 37 mmol/L KCl, 1.5 mmol/L MgCl2, 10 mmol/L DTT, 0.25 mmol/L dNTP, 40 units of RNAsin, 50 U/mL Super Taq DNA polymerase, and 200 units of reverse transcriptase. The annealing mixture was incubated at room temperature for 15 min, and then incubated in water bath at 41 °C for 60 min. The reverse transcriptase enzyme was inactivated by heating the solution to 95 °C for 5 min. Thirty cycles of PCR were carried out using the Perkin-Elmer PCR reaction kit and primers, each consisting of denaturation at 94 °C for 1 min, annealing at 57 °C for 1 min, and extension at 72 °C for 2 min. PCR products were separated on 2% agarose gel. Primers used for PCR were p21 sense (5’-GGG GAC AGC AGA GGA AGA C-3’) and p21 antisense (5’-CGG CGT TTG GAG TGG TAG A-3’), β-actin sense (5’-GAT TGC CTC AGG ACA TTT CTG- 3’) and β-actin antisense (5’-GAT TGC TCA GGA CAT TTC TG-3’). Gene primers were synthesized by Beijing Oake Co.
Student’s t-test was used to compare the difference between control group and allitridi treatment group. The data of cell growth and mitotic index were presented as mean盨D. P value less than 0.05 was considered statistically significant.
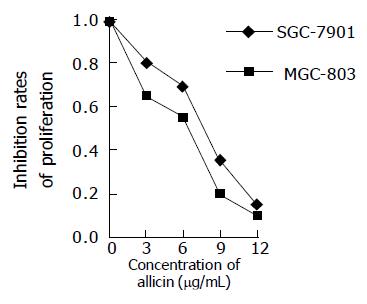
Figure 1 shows the proliferation curves of the cells treated with allitridi at various concentrations. The proliferation of MGC803 and SGC7901 cells inhibited by allitridi was clearly observed in a concentration-dependent manner during the 24-h period. MGC803 cell growth was inhibited by allitridi with 24 h IC50 being 6.4 μg/mL. SGC7901 cell growth was inhibited by allitridi with 24 h IC50 being 7.3 μg/mL.
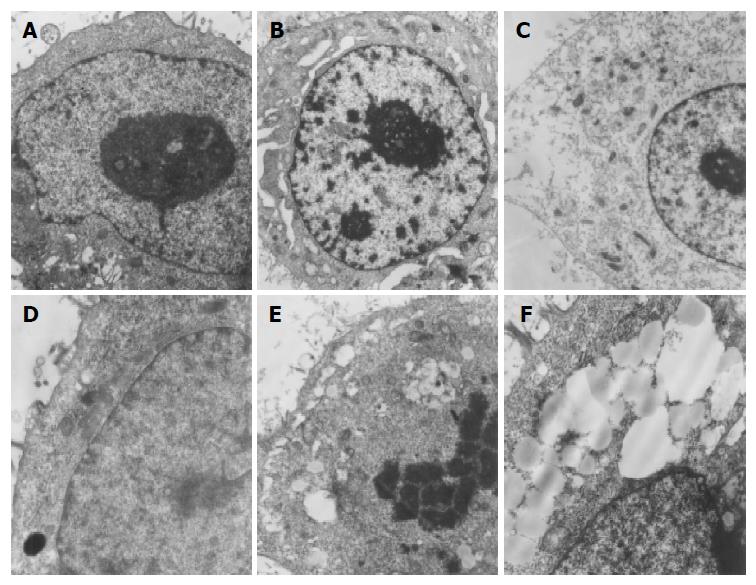
After being treated with allitridi at the concentration of 12 μg/mL for 24 h, cell morphological change was observed under transmission electron microscope. The ultrastructure showed that the cells were directly killed and broken cellular membrane, swollen and vesiculated mitochondria and rough endoplasmic reticula and mass lipid droplet could be seen. The nuclei had no obvious morphological change or typical apoptosis (Figure 2).
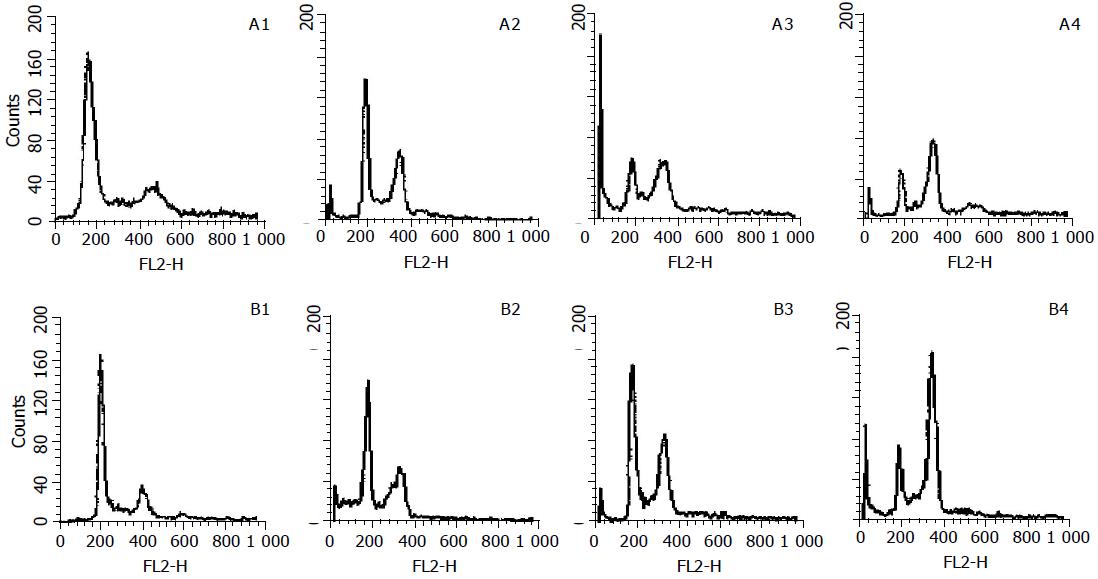
To assess the effects of allitridi on cell cycle progression and population distribution, MGC803 and SGC7901 cells were studied using flow cytometry. A comparison of the cell cycle profiles of allitridi treatment and control cells confirmed the existence of allitridi-induced effects. It was found that 24-h allitridi treatment induced significant cell cycle arrest in G2/M phase (P<0.01) in comparison to untreated cells. Histograms are shown in Figure 3. The percentage of cells in the S phase, G1, and G2/M regions are shown in Table 1. After being treated with allitridi at the concentration of 6 μg/mL for 24 h, cell mitotic index was determined. The mitotic index of MGC803 was 316 after allitridi treatment, and 21 in control group. The mitotic index of SGC7901 was 207 after allitridi treatment, and 16 in control group. Compared with the control group, the cell mitotic index of allitridi treatment group was much higher (P<0.01), indicating that allitridi caused potential M phase blockage in gastric cancer cells.
| MGC803 | SGC7901 | |||||||
| Control | Treated with allitridi | Control | Treated with allitridi | |||||
| 3 μg/mL | 6 μg/mL | 9 μg/mL | 3 μg/mL | 6 μg/mL | 9 μg/mL | |||
| G0/G1 (%) | 57.2±3.1 | 27.5±2.8b | 12.4±1.7b | 3.3±0.6b | 62.1±2.4 | 27.7±1.0b | 17.9±3.8b | 13.2±1.7b |
| S (%) | 14.4±1.6 | 13.6±1.4 | 12.4±2.2 | 11.2±1.3 | 10.1±0.5 | 12.3±0.1 | 13.7±2.1 | 12.1±0.4 |
| G2/M (%) | 13.3±0.2 | 20.1±3.5b | 36.5±1.4b | 36.4±4.5b | 13.2±1.2 | 21.7±2.3b | 39.4±4.3b | 37.4±3.3b |
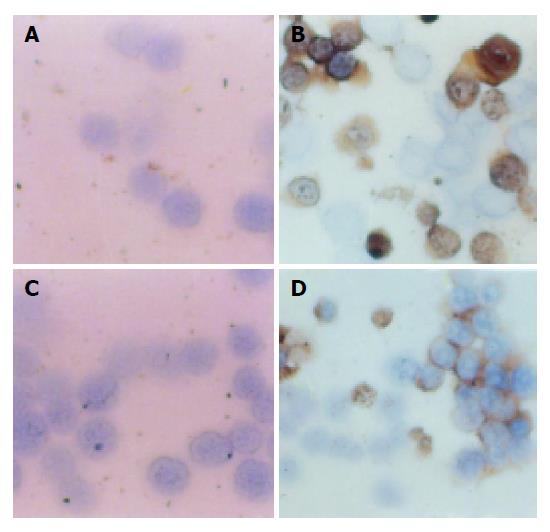
The effect of allitridi on p21 protein levels was determined by immunohistochemistry analysis. p21 was not expressed in MGC803 and the SGC7901 cells. However, when MGC803 cells were treated with allitridi at the concentrations of 3, 6, and 9 μg/mL for 24 h, p21 was expressed in high levels, the positive rates of p21 were 28.2%, 29.1%, and 31.8% respe-ctively. Similarly when SGC7901 cells were treated under exactly same as above, p21 expressed in high levels too, the positive rates of p21 were 25.5%, 35.1%, or 39.6%. Figure 4 shows the levels of p21 in MGC803 and SGC7901 cells.
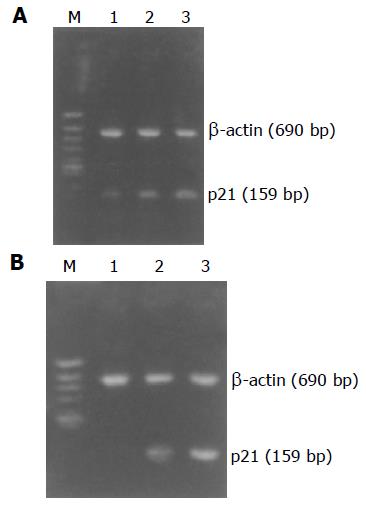
p21WAF1 mRNA expressed little in MGC803 and SGC7901 cells. However, we demonstrated that treatment with 6 or 9 μg/mL allitridi did induce upregulation of p21WAF1 mRNA in MGC803 and SGC7901 cells. In this study, RT-PCR products of β-actin were used as an inter control. Figures 5A and B show that treatment of MGC803 and SGC7901 cells with allitridi induced an upregulation of p21WAF1.
Allitridi containing DATS and DADS is widely used in cancer chemoprevention and anti-cardiovascular disease research[4-6]. Some scholars have reported its protective effects on HGC. DATS can significantly inhibit bacterial nitrosamines. The mechanism is that the constituent of garlic can diminish NO2, thus preventing the formation of carcinogens[7]. Cellular immune response of patients treated with allitridi shows that the macrophage phagocytotic ability and lymphocyte transformation percentage are obviously increased in most patients. Cell cycle plays an important role in the modulation of growth of tumor cell, and attention has been paid to preventing unlimited proliferation of tumor cells by cell cycle control[8]. In this study, the proliferation of human gastric cell lines MGC803 and SGC7901 was significantly inhibited after being treated with allitridi at different concentrations. Morphological analysis demonstrated direct cytotoxic effects of allitridi on tumor cells. The cells were detected using flow cytometer in order to examine the distribution of DNA content. The first peak (2C C = haploid DNA content) was produced by the cells in G1 phase, the second (4C) was produced in G2/M phase with or without cells in G1 phase at higher DNA ploidy (tetraploidy, G1t), and the third (8C) was produced in G2 phase at higher DNA ploidy (tetraploidy, G2t). The results show that the cells are blocked in G2/M phase. Furthermore, after allitridi treatment, cell mitotic index was much higher, the cell cycle was blocked just in M phase, indicating that allitridi makes damaged cells stagnate at mitosis phase of cell cycle, and inhibits the proliferation of cells. If this happens, cells re-enter the cycle, otherwise they undergo apoptosis or death. More importantly, allitridi affects M phase of these cells selectively and participates in the regulation of cell cycle, and the blocking effect of allitridi on cells in M phase could be the mechanism of its antitumor effect. Allitridi can be used as a chemotherapeutic agent. Thus, the goal in cancer therapy is to kill cancer cells. Cell cycle control offers a new set of potential target for chemotherapeutic compounds, many of these new agents may be biological modifiers, rather than nonselective cytotoxic agents. The results of the present study reveal that MGC803 and SGC7901 cells have low p21 expression, but allitridi increases the expression of p21WAF1 of MGC803 and SGC7901 cells. Completion of the cell cycle requires the co-ordination of a variety of macromolecular syntheses, assemblies, and movements. Negative controls on cell cycle progression are exerted during cell development, differentiation, senescence, and death. These negative controls may play an important role in preventing tumorigenesis. p21WAF1 has been implicated as a growth arrest mediator in cell terminal differentiation and apoptosis[9,10]. Regulation of cell cycle progression is orchestrated by CDKs.
p21WAF1 is a downstream effector of p53 that mediates cell cycle arrest in G1 and G2/M phases. Mechanically, the p21WAF1-mediated cell cycle arrest in the G1 and G2/M phases has been suggested to include p21WAF1-CDK2 and p21WAF1-PCNA protein interaction[11]. As we have known, p21 is the cell cycle profiling, when overexpressed it inhibits the CDK4/6-cyclinD complex, leading to cell growth arrest and apoptosis[12], suggesting that many such control points exist within the cell cycle and they play a major role in maintaining the integrity of genome. At least two checkpoints detect DNA damage, one at the G-S transition, the other at the G2-M transition. In addition, there are mitotic checkpoints, altered expression of cyclins A, B, and CDC2. All potential targets of mitotic checkpoint controls occur in some cancers[13]. Allitridi can upregulate p21WAF1. P21 can affect the growth of cells by repressing M phase, and may interact with Cdc2-cyclinB mitosis promoting complex and inhibit its kinase activity; at this point it needs to be further investigated.
In conclusion, allitridi can effectively inhibit the proliferation of HGC MGC803 and SGC7901 cells and upregulate the expression of p21WAF1. This study provides experimental data for clinical use of allitridi in treatment of gastric carcinoma.
We are deeply grateful to Dr. Yun-Peng Liu, Director of Department of Internal Oncology, First Affiliated Hospital of China Medical University for providing MGC803 and SGC7901 cells suggestions and reading of the manuscript. We also thank Kezuo Hou for skillful technical inst-ruction.
Science Editor Wang XL and Yu DH Language Editor Elsevier HK
| 1. | Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr. 2000;72:1047-1052. [PubMed] [Cited in This Article: ] |
| 2. | Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H: quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun. 1998;244:917-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Balasenthil S, Arivazhagan S, Ramachandran CR, Nagini S. Effects of garlic on 7,12-Dimethylbenz [a] anthracene-induced hamster buccal pouch carcinogenesis. Cancer Detect Prev. 1999;23:534-538. [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Wartenberg M, Fischer K, Hescheler J, Sauer H. Modulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by cell cycle inhibitors. Biochim Biophys Acta. 2002;1589:49-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Zeng YX, el-Deiry WS. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene. 1996;12:1557-1564. [PubMed] [Cited in This Article: ] |
| 6. | Wu Q, Kirschmeier P, Hockenberry T, Yang TY, Brassard DL, Wang L, McClanahan T, Black S, Rizzi G, Musco ML. Transcriptional regulation during p21WAF1/CIP1-induced apoptosis in human ovarian cancer cells. J Biol Chem. 2002;277:36329-36337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Bertrand R, Solary E, O'Connor P, Kohn KW, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211:314-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 397] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Lin SY, Liang YC, Ho YS, Tsai SH, Pan S, Lee WS. Involvement of both extracellular signal-regulated kinase and c-jun N-terminal kinase pathways in the 12-O-tetradecanoylphorbol-13-acetate-induced upregulation of p21(Cip1) in colon cancer cells. Mol Carcinog. 2002;35:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Prall OW, Carroll JS, Sutherland RL. A low abundance pool of nascent p21WAF1/Cip1 is targeted by estrogen to activate cyclin E*Cdk2. J Biol Chem. 2001;276:45433-45442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Javelaud D, Besancon F. Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J Biol Chem. 2002;277:37949-37954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Chen B, He L, Savell VH, Jenkins JJ, Parham DM. Inhibition of the interferon-gamma/signal transducers and activators of transcription (STAT) pathway by hypermethylation at a STAT-binding site in the p21WAF1 promoter region. Cancer Res. 2000;60:3290-3298. [PubMed] [Cited in This Article: ] |
| 12. | Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expressionand gene-associated histone acetylation. Cell Biol. 2000;97:10014-10019. [Cited in This Article: ] |
| 13. | Kovalsky O, Lung FD, Roller PP, Fornace AJ. Oligomerization of human Gadd45a protein. J Biol Chem. 2001;276:39330-39339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |