Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3465
Revised: July 28, 2004
Accepted: November 29, 2004
Published online: June 14, 2005
AIM: To determine the validity of the non-invasive method of CT perfusion (CTP) in rat model of hepatic diffuse disease.
METHODS: Twenty-eight Wistar rats were divided into two groups. Liver diffuse lesions were induced by diethyln-itrosamine in 14 rats of test group. Rats in control group were bred with pure water. From the 1st to 12th wk after the test group was intervened, both groups were studied every week with CTP. CTP parameters of liver parenchyma in different periods and pathologic changes in two groups were compared and analyzed.
RESULTS: The process of hepatic diffuse lesions in test groups was classified into three stages or periods according to the pathologic alterations, namely hepatitis, hepatic fibrosis, and cirrhosis. During this period, hepatic artery flow (HAF) of control group declined slightly, mean transit time (MTT), blood flow (BF) and volume (BV) increased, but there were no significant differences between different periods. In test group, HAF tended to increase gradually, MTT prolonged obviously, BV and BF decreased at the same time. The results of statistical analysis revealed that the difference in the HAF ratio of test group to control group was significant. The ratio of BV and BF in test group to control group in stage of hepatitis and hepatic cirrhosis, hepatic fibrosis and early stage of hepatic cirrhosis was significantly different, but there was no significant difference between hepatitis and hepatic fibrosis. The main pathological changes in stage of hepatitis were swelling of hepatic cells, while sinusoid capillarization and deposition of collagen aggravated gradually in the extravascular Disse’s spaces in stage of fibrosis and early stage of cirrhosis.
CONCLUSION: The technique could reflect some early changes of hepatic blood perfusion in rat with liver diffuse disease and is valuable for their early diagnosis.
- Citation: Guan S, Zhao WD, Zhou KR, Peng WJ, Mao J, Tang F. CT perfusion at early stage of hepatic diffuse disease. World J Gastroenterol 2005; 11(22): 3465-3467
- URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3465
The imaging diagnosis of hepatitis, hepatic fibrosis and early-stage hepatic cirrhosis is still very difficult because they lack morphological changes. However, some hemodynamic changes of these diseases have been found[1]. Invasive and non-invasive methods[2-4] are employed to detect these alterations. At present, blood perfusion data of liver tissues and lesions can be obtained by non-invasive method of CT perfusion (CTP) scan using rapid scan technique and analytic software. Clinical observations have shown its feasibility[5-7]. But there are few studies on the blood changes in hepatitis, hepatic fibrosis and cirrhosis at early stage[8]. This study was to investigate the validity of CTP in rat model of hepatic diffuse disease.
Twenty-eight male Wistar rats, 6 wk old, were divided into test and control groups (14 each group). Three days were given to get themselves adapted to the environment in independent ventilating cabinets. Test group was continually intervened every day by adding diethylnitrosamine (DEN, 0.01%, Sigma, 0.99 mg/mL) into their drinking water. Control group drank pure water at the same time. The rats in both groups were scanned by spiral CT once a week for 12 wk after the intervention in test group. Rats of both groups were killed 24 h after CT scan and liver parenchyma tissue of corresponding part was collected and stained with hematoxylin and eosin for pathological examination.
All examinations were completed on a spiral CT scanner. Plain scan was performed to select a slice in which the liver, aorta and portal vein were clearly visualized. Then single section sequential dynamic contrast enhancement CT scan was done according to the protocol of 0.6 s scan time, 0.4 s cycle time, 50 s total scan time; 120 kV, 80 mA; 5-mm slice, 512×512 matrix, 12 cm FOV. An ionic contrast agent (60% angiografin, 1 mL/kg) with diluted concentration of 33% was injected into the tail vein after 5-10 slices were scanned.
Within 24 h after CT imaging, the rats were killed and livers were removed at autopsy. DEN-induced liver injury was assessed by standard hematoxylin-eosin staining and electron microscopy, and compared to the normal livers.
All images were transmitted to a workstation (SPARC; Volume Viewer) for data analysis using GE AW4.2. Four of the six parameters were calculated by this software, including blood flow (BF), blood volume (BV), mean transit time (MTT), hepatic arterial flow (HAF). The other two parameters devised for tumor lesion were not included in this test. Three regions of interest (ROI) were drawn in the abdominal aorta, portal vein and right lobe of the liver respectively to obtain satisfactory time-density curves. Hepatic artery was substituted by abdominal aorta because of its small caliber. The ROI of liver parenchyma was as large as possible and avoided large blood vessels.
Data were expressed as mean±SD. The Student-Newman-Keuls was used for comparison between two groups in different periods. Considering the changes of perfusion parameters in normal group, ratios of the relative data between test group and normal group were compared. P<0.05 was considered statistically significant.
Hepatic lesions in test group were proved to be the main pathologic changes manifested as swelling of hepatocytes 1-4 wk after intervention. Fibrotic changes were found at 5-8 wk. Hepatic lesions developed into cirrhosis at 9-14 wk and were characterized by many cirrhosis nodules. Diffuse lesions in different lobes were at different levels. Left lobe and/or left segment of right lobe developed into cirrhosis earlier and more serious than others.
No obvious morphologic changes were seen in both groups during the experiment.
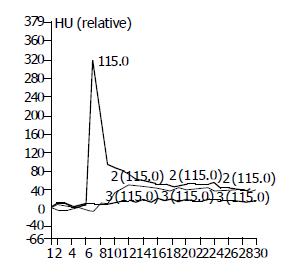
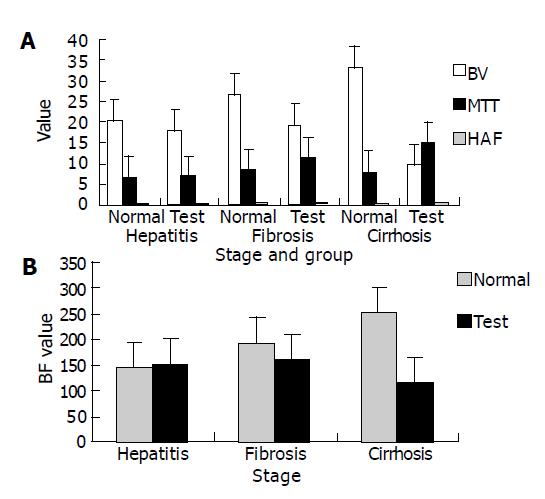
Time-density curves (Figure 1) of CTP were satisfactory and all hepatic blood perfusion data (Table 1) could be obtained. From the 1st to 12th wk after the test group was treated with DEN, HAF increased gradually, MTT prolonged obviously, BV and BF decreased at the same time. While HAF of control group declined slightly, MTT, BF and BV increased. Statistical analysis (Figure 2) showed that the differences in the HAF ratios of test group to control group at different stages were significant (P<0.05). The BV and BF ratios at stage of hepatitis and hepatic cirrhosis, hepatic fibrosis and early stage of hepatic cirrhosis were significantly different (P<0.05), but no significant difference was found between hepatitis and hepatic fibrosis.
| Hepatitis (1-4 wk) | Fibrosis (5-8 wk) | Cirrhosis (9-12 wk) | ||||
| Test | Normal | Test | Normal | Test | Normal | |
| HAF (%) | 0.33±0.23 | 0.25±0.17 | 0.55±0.13 | 0.22±0.16 | 0.7±0.24 | 0.19±0.17 |
| BV (mL/100 g/min) | 18.05±3.27 | 20.54±2.35 | 19.35±3.96 | 26.65±6.67 | 9.51±3.61 | 33.06±4.45 |
| BF (mL/100 g) | 152.84±59.12 | 143.43±26.65 | 161.77±56.64 | 192.44±79.98 | 117.59±78.66 | 251.67±73.50 |
| MTT (s) | 6.6±2.39 | 6.57±3.28 | 11.41±3.92 | 8.3±2.75 | 15.02±5.21 | 7.88±2.06 |
At present, the diagnosis of hepatitis, hepatic fibrosis and early hepatic cirrhosis in clinic mainly depends on blood examination and the history of patients. Hepatic biopsy has to be considered in order to determine the degree of lesion but is not always exact. Hemodynamic change is important in determining the function of liver. The blood volume of liver accounting for 1/4 of cardiac output is important in maintaining hepatic function. Decrease of blood perfusion in liver will impair its function by reducing the exchange of blood and hepatocytes. Therefore, the investigation of perfusion parameters of liver is of great value in clinical diagnosis and treatment of liver disease.
Non-invasive techniques used in clinic to estimate hepatic perfusion include hepatic clearance of sorbitol, SPECT, PET, Doppler, etc. However, there are some shortcomings of these methods. For example, the method of hepatic clearance of sorbitol is affected severely by some enzymes and transit mechanism when applied in patients with severe liver diffuse disease. Nuclear medicine techniques are hampered by their limited spatial and temporal resolution in differentiating the overlapping parts of hepatic artery and portal vein perfusion in liver, and can barely reflect a large area of liver and can be affected easily by abdominal aorta, inferior vena cava and right kidney when hepatic artery perfusion is measured quantitatively[3]. Doppler and color Doppler can only measure the velocity of large blood vessels and could not directly evaluate the blood amount of hepatic parenchyma[9]. The applications of invasive methods including angiography, CTA, CTAP, etc., do not play an important role in routine clinical practice.
Studies of Miles et al[10], reported that it is possible to analyze hepatic perfusion using single slice dynamic CE CT scan. According to its achievements in brain diseases[11], this technique may be a promising way to detect hepatic blood perfusion. To date, there are few reports about its application in liver diseases[12,13].
Our results showed that HAF in control group declined slightly and MTT, BF and BV increased gradually from the 1st to 12th wk after the test group was treated, but there were no significant differences. The explanation may be because the experimental rats were growing and hepatic perfusion data would change with the growth. In test group, HAF tended to increase, MTT prolonged obviously, BV and BF decreased at the same time. Considering the changes of perfusion parameters in control group, the ratios of the counterpart data in test group and control group were compared. The results of statistical analysis demonstrated that the differences in HAF and MTT at different stages of test group were significant (P<0.05), and the differences in BV and BF between hepatitis and hepatic cirrhosis, hepatic fibrosis and early stage of hepatic cirrhosis were significant (P<0.05), suggesting that single slice dynamic CE CT scan can estimate the changes of hepatic blood perfusion related with liver diffuse disease. There was no significant difference in BV and BF between hepatitis and hepatic fibrosis. The explanation may be that liver at this stage can still compensate for its blood perfusion by increasing hepatic arterial volume or the pressure of portal vein. The perfusion data of early stage of hepatic cirrhosis changed a lot compared to those at stage of hepatitis. Pathologic analysis showed that transformation of the fenestrated sinusoids into continuous capillaries and deposition of collagen in the extravascular spaces were the key factors. This kind of alterations may increase the interstitial pressure and obstruct hepatic blood perfusion. Shunts between hepatic artery and portal vein disturb hepatic perfusion in hepatic cirrhosis, but this phenomenon was not obvious in our animal models.
MR perfusion in liver has been studied recently. However, there is no good analytic software because of its complexity compared to CT. Furthermore, the theory and manipulation of MR perfusion are not as simple as CTP in liver examination. Therefore, CTP is more feasible than MR at present.
We thank Hong Ao for the establishment of rat model; Guang Cao, Fei Sun, Wei Sun for the MR scan; Zhong-Wei Chen for the pathologic analysis.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Orrego H, Blendis LM, Crossley IR, Medline A, Macdonald A, Ritchie S, Israel Y. Correlation of intrahepatic pressure with collagen in the Disse space and hepatomegaly in humans and in the rat. Gastroenterology. 1981;80:546-556. [PubMed] |
| 2. | Nakao N, Miura K, Takahashi H, Miura T, Ashida H, Ishikawa Y, Utsunomiya J. Hepatic perfusion in cavernous transformation of the portal vein: evaluation by using CT angiography. AJR Am J Roentgenol. 1989;152:985-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | O'Connor MK, Krom RF, Carton EG, Sanchez-Urdazpal L, Juni JE, Ferguson DM, Wiesner RF. Ratio of hepatic arterial-to-portal venous blood flow--validation of radionuclide techniques in an animal model. J Nucl Med. 1992;33:239-245. [PubMed] |
| 4. | Annet L, Materne R, Danse E, Jamart J, Horsmans Y, Van Beers BE. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Leggett DA, Kelley BB, Bunce IH, Miles KA. Colorectal cancer: diagnostic potential of CT measurements of hepatic perfusion and implications for contrast enhancement protocols. Radiology. 1997;205:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Bader TR, Herneth AM, Blaicher W, Steininger R, Mühlbacher F, Lechner G, Grabenwöger F. Hepatic perfusion after liver transplantation: noninvasive measurement with dynamic single-section CT. Radiology. 1998;209:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Arita T, Matsunaga N, Takano K, Hara A, Fujita T, Honjo K. Hepatic perfusion abnormalities in acute pancreatitis: CT appearance and clinical importance. Abdom Imaging. 1999;24:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 259] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 9. | Kok T, Slooff MJ, Peeters PM, Zwaveling JH, Bijleveld CM, Gi-van Loon CE, Klompmaker IJ, Haagsma EB. Changes in portal hemodynamics and acute rejection in the first 2 weeks after orthotopic liver transplantation. A prospective Doppler ultrasound study. Invest Radiol. 1996;31:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 263] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Wintermark M, Smith WS, Ko NU, Quist M, Schnyder P, Dillon WP. Dynamic perfusion CT: optimizing the temporal resolution and contrast volume for calculation of perfusion CT parameters in stroke patients. AJNR Am J Neuroradiol. 2004;25:720-729. [PubMed] |
| 12. | Tsushima Y, Funabasama S, Sanada S, Aoki J, Endo K. Perfusion changes of hepatic parenchyma due to infectious hepatobiliary disease: demonstration by perfusion CT. Comput Med Imaging Graph. 2003;27:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Shi LJ, Tian JM, Wang PJ, Bi YM, Tian J, Li SP, Li YL. Pilot study on clinical application of hepatic perfusion with multi-slice spiral CT. Zhonghua GanZangBing ZaZhi. 2003;11:522-525. [PubMed] |