INTRODUCTION
In 1998, star-shaped cells in the pancreas, namely pancreatic stellate cells (PSCs), were identified and characterized[1,2]. In normal pancreas, stellate cells are quiescent and can be identified by the presence of vitamin A-containing lipid droplets in the cytoplasm. In response to pancreatic injury or inflammation, they are transformed (“activated”) from their quiescent phenotype into myofibroblast-like cells, which actively proliferate, express the cytoskeletal protein α-smooth muscle actin, and produce extracellular matrix components. Many of the morphological and metabolic changes associated with the activation of PSCs in animal models of fibrosis also occur when these cells are grown in serum-containing medium in culture on plastic. There is accumulating evidence that activated PSCs play a pivotal role in the development of pancreatic fibrosis[1-4]. In addition, PSCs may participate in the pathogenesis of acute pancreatitis[3,5]. The activation of signaling pathways such as p38 mitogen-activated protein (MAP) kinase[6], Rho-Rho kinase[7], and c-Jun N-terminal kinase[8] is likely to play a role in PSC activation.
Stellate cell proliferation and expansion of their pool are the fundamental features of pancreatic fibrosis[3]. Platelet-derived growth factor (PDGF)-BB has been shown to be one of the most potent mitogen of PSCs, and is likely to be an important mediator of the increased proliferation of PSCs both in vivo and in vitro[9,10]. Accumulation of PSCs may also result from PSC migration, and recent studies have shown that PDGF-BB induced migration of PSCs[11,12]. Binding of PDGF to the receptors leads to dimerization of receptor subunits, phosphorylates the receptor on tyrosines (known as “autophosphorylation”), changes its cytoplasmic conformation, activates endogenous tyrosine kinases, and initiates intracellular signaling[13,14]. For downstream of PDGF receptor, there are at least two major signaling pathways: phosphatidylinositol 3-kinase (PI3-kinase)/Akt and c-Raf/MAP kinase kinase/extracellular signal-regulated kinase (ERK) pathways[13,14]. We and others have shown that ERK pathway contributes mainly to cell proliferation whereas PI3-kinase/Akt pathway contributes to cell migration[11,15]. Regulation of PSC proliferation and migration would serve as a therapeutic target for pancreatic fibrosis and inflammation.
Natural antioxidants, such as polyphenols from green tea extracts, have attracted considerable attention for the prevention of oxidative stress-related diseases including cancer, cardiovascular diseases, and degenerative diseases[16,17]. Of the polyphenols purified from green tea, (-)-epigallocatechin-3-gallate (EGCG) is a major constituent and the most potent antioxidant[18]. The antioxidant potential of EGCG is far greater than that of vitamin E and/or C[19]. Previous studies have demonstrated that EGCG possesses antioxidant[20], anti-inflammatory[21], anti-proliferative[22], and anti-cancer activities[23-26]. EGCG inhibited lipopolysaccharide-induced nitric oxide production and inducible nitric oxide synthase gene expression in isolated peritoneal macrophages by decreasing the activation of nuclear factor κB[21]. EGCG inhibited PDGF-induced proliferation of vascular smooth muscle cells[22]. EGCG induced apoptosis more readily in cancer cells than their natural counterparts[23]. EGCG inhibited tumor growth, metastasis, and angiogenesis in vivo[24-26]. But no previous studies have addressed the effects of EGCG on cell functions of PSCs. We here report that EGCG inhibited PDGF-induced proliferation and migration of PSCs through the inhibition of PDGF-mediated signaling.
MATERIALS AND METHODS
Materials
EGCG was dissolved in H2O and stocked at 10 mmol/L. 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was obtained from Dojindo (Kumamoto, Japan). Rat recombinant PDGF-BB was purchased from R&D Systems (Minneapolis, MN). Rabbit antibodies against ERK (phosphorylated and total) and Akt (phosphorylated and total) were purchased from Cell Technologies, Inc. (Beverly, MA). Rabbit antibodies against PDGF β-receptor, cyclin D1, p21Waf1, and p27Kip1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Trevigen (Gaithersburg, MD). Rabbit antibody against phosphorylated PDGF β-receptor and mouse anti-phosphotyrosine antibody were obtained from Upstate Biotechnology Inc. (Lake Placid, NY). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specifically described.
Cell culture
All animal procedures were performed in accordance with the National Institutes of Health Animal Care and Use Guidelines. Rat PSCs were prepared from the pancreas tissues of male Wistar rats (Japan SLC Inc., Hamamatsu, Japan) weighting 200-250 g using the Nycodenz solution (Nycomed Pharma, Oslo, Norway) after perfusion with 0.3 g/L collag-enase P as previously described[11]. The cells were resuspended in Ham’s F-12 medium containing 100 mL/L heat-inactivated fetal bovine serum (MP Biomedicals, Irvine, CA), penicillin sodium, and streptomycin sulfate. Cell purity was always more than 90% as assessed by a typical star-like configuration and by detecting vitamin A autofluo-rescence. All experiments were performed using cells between passages two and five. We incubated PSCs in serum-free medium for 24 h before the addition of experimental reagents. EGCG was added to the culture medium 1 h before the addition of PDGF-BB.
Cell viability assay
Cell viability was assessed by the MTT assay as previously described[27]. After the treatment with EGCG at the indicated concentrations for 72 h, MTT solution was added to the cells at a final concentration of 500 mg/L, and the incubation continued at 37 °C for 4 h. After the incubation period, the medium was aspirated and the formazan product was solubilized with dimethylsulfoxide. Cell viability was determined by A570-690.
Cell proliferation assay
Serum-starved PSCs (approximately 80% density) were treated with PDGF-BB (at 25 μg/L) in the presence of EGCG at the indicated concentrations. Cell proliferation was assessed using a commercial kit (Cell proliferation ELISA, BrdU; Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instruction. This is a colorimetric immunoassay based on the measurement of 5-bromo-2’-deoxyuridine (BrdU) incorporation during DNA synthesis. After 24-h incubation with experimental reagents, cells were labeled with BrdU for 3 h at 37 °C. Cells were fixed, and incubated with peroxidase-conjugated anti-BrdU antibody. Then the peroxidase substrate 3,3’,5,5’-tetramethylbenzidine was added, and BrdU incorporation was quantitated by A370-492.
Cell migration assay
Cell migration was assessed as previously described[11]. Serum-starved PSCs were trypsinized, and resuspended at the concentration of 3×108 cells/L in serum-free medium containing EGCG at the indicated concentrations. For the assay, we used modified Boyden chambers with 8-μm-pore filters (Iwaki glass Co. Ltd., Funabashi, Japan) coated with rat-tail type I collagen. PDGF-BB (at 25 μg/L) was added to the lower chamber, and 250 μL of cell suspension was added to the upper chamber. The chambers were then incubated at 37 °C for 24 h. At the end of the incubation, the cell suspension in the upper chamber was aspirated, and the upper part of the filter was cleaned with cotton plugs. The cells migrated to the underside of the filter were stained with Difquick (Sysmex, Kobe, Japan), viewed, and counted at 200× magnification.
Cell cycle analysis
The cell cycle of PSCs was analyzed by flow cytometry as previously described[28]. Briefly, serum-deprived PSCs (approximately 60-70% density) were treated with PDGF-BB in the absence or presence of EGCG (at 25 μmol/L). After 24 h, cells were harvested and washed twice with phosphate-buffered saline. Cells were suspended in phosphate-buffered saline solution containing 40 mg/L propidium iodide, 0.2 mL/L Triton X-100, and 50 mg/L ribonuclease A. Samples were incubated in the dark at room temperature for 30 min and stored at 4 °C until the analysis. Cell fluorescence was measured by FACSCaliber flow cytometer (Becton Dickinson Co. Ltd, Tokyo, Japan), and analyzed using ModFit LT software (Verity Software House, Topsham, ME) to determine the distribution of cells in the various phases of the cell cycle.
Western blotting
The level of activated, phosphorylated ERK was determined by Western blotting as previously described[29]. Cells were lysed in sodium dodecyl sulfate buffer. Cellular proteins (approximately 100 μg) were fractionated on a 100 g/L sodium dodecyl sulfate-polyacrylamide gel. They were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), and the membrane was incubated overnight at 4 °C with rabbit anti-phosphospecific ERK antibody. After incubation with peroxidase-conjugated goat anti-rabbit IgG antibody for 1 h, proteins were visualized using an ECL kit (Amersham Biosciences UK Ltd). Levels of total ERK, Akt (phosphorylated at Ser473 and total), PDGF β-receptor (phosphorylated at Tyr716 and total), cyclin D1, p21Waf1, p27Kip1, and GAPDH were determined in a similar manner.
PI3-kinase assay
Serum-starved PSCs were treated with EGCG at the indicated concentrations in the absence or presence of PDGF-BB. After 5-min incubation, the monolayer was lysed in modified radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl at pH 7.4, 10 mL/L Nonidet P-40, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L activated sodium orthovanadate, 1 μmol/L phenylmethylsulfonyl fluoride, and 1 mg/L of each aprotinin, leupeptin, and pepstatin). The samples were centrifuged at 12000 g for 5 min to remove insoluble cell debris. The protein concentration in the supernatant was determined using the BCA protein assay (Pierce, Rockford, IL). Cell lysates (approximately 250 μg) were incubated with the anti-phosphotyrosine antibody overnight at 4 °C. The immune complex was absorbed to protein A-agarose beads (Upstate Biotechnology Inc.) for 2 h at 4 °C. PI3-kinase activity was determined using PI as a substrate as previously described [30]. The product, PI3-phosphate, was resolved by thin layer chromatography in chloroform: methanol:water:300 g/L ammonium hydroxide (60:47:11.3:2, v/v) as a solvent. After drying, the plates were autoradiographed. Unlabeled PI3-phosphate was run in parallel to determine its position.
Statistical analysis
The results were expressed as mean±SD. Luminograms and autoradiograms are representative of at least three experiments. Differences between the groups were evaluated by ANOVA, followed by Fisher’s test for post hoc analysis. A P-value less than 0.05 was considered statistically significant.
RESULTS
EGCG was cytotoxic at higher concentrations
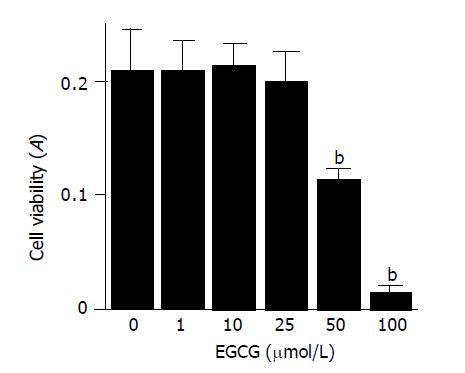
We first examined the effect of EGCG on the cell viability of PSCs. PSCs were incubated with increasing concentrations of EGCG in serum-free medium for 72 h, and the cell viability was assessed by MTT assay (Figure 1). EGCG up to 25 μmol/L did not alter the cell viability, but at higher concentrations, EGCG was cytotoxic to PSCs (Figure 2). The results were also confirmed by trypan blue dye exclusion test (data not shown). Based on these results, we used EGCG up to 25 μmol/L in the subsequent experiments.
Figure 1 Chemical structure of EGCG.
Figure 2 EGCG was cytotoxic at higher concentrations.
PSCs were treated with EGCG at the indicated concentrations (μmol/L) in serum-free medium for 72 h. Cell viability was determined by the MTT assay, and the absorbance at 570-690 nm (“A”) of the sample is shown. Data are shown as mean±SD (n = 6). bP<0.01 vs EGCG at 0 μmol/L. A: optical density.
EGCG inhibited PDGF-induced proliferation and migration of PSCs
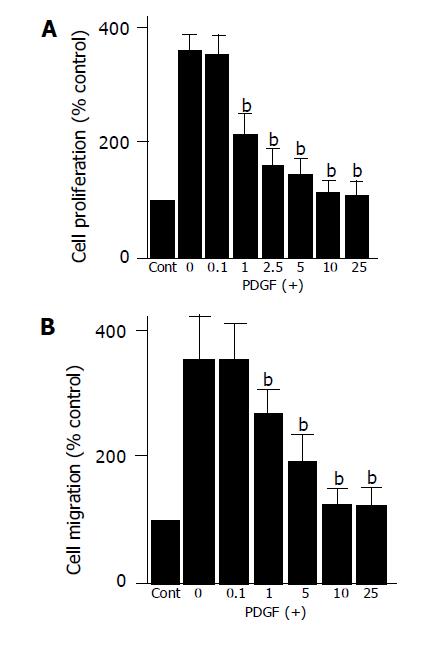
In agreement with the previous studies showing that PDGF-BB is a potent mitogen of PSCs in vitro[9,10], PDGF-BB significantly increased proliferation of PSCs (Figure 3A). PDGF-induced proliferation was inhibited by EGCG in a dose-dependent manner. The inhibitory effect was significant at as low as 1 μmol/L.
Figure 3 EGCG inhibited PDGF-induced proliferation and migration.
A: Serum-starved PSCs were left untreated (“Cont”) or treated with PDGF-BB (at 25 μg/L) in the presence or absence of EGCG at the indicated concentrations (μmol/L). After 24-h incubation, DNA synthesis was assessed by BrdU incorporation enzyme-linked immunosorbent assay. Data are shown as mean±SD (% of the control, n = 6). bP<0.01 vs PDGF only; B: cell migration was assessed using modified Boyden chambers with 8-μm pore filters. Serum-starved PSCs were left untreated (“Cont”) or were treated with PDGF-BB (at 25 μg/L) in the lower chamber in the absence or presence of EGCG at the indicated concentrations (μmol/L). After 24-h incubation with PDGF, the cells migrated to the underside of the filter were stained, and counted. Data are shown as mean±SD (% of the control, n = 6). bP<0.01 vs PDGF only.
Accumulation of PSCs in fibrotic pancreas may also result from PSC migration, and PDGF-BB has been shown to be a potent inducer of PSC migration[11,12]. We examined whether EGCG affected PDGF-induced migration of PSCs. EGCG inhibited PDGF-BB-induced PSC migration in a dose-dependent manner (Figure 3B).
EGCG inhibited cell cycle progression beyond G1 phase
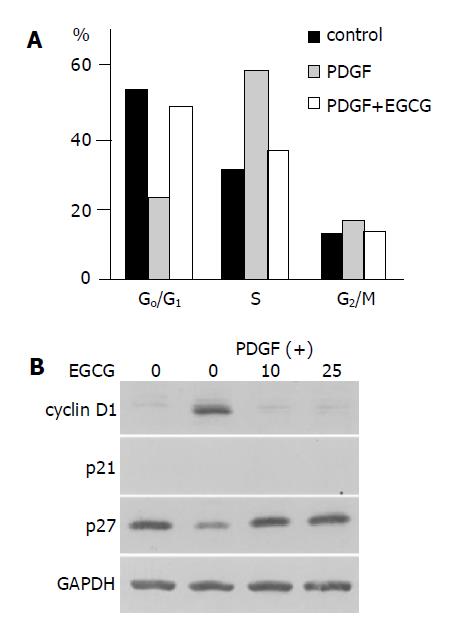
We analyzed the cell cycle in PSCs in the presence or absence of EGCG. Exposure to PDGF was associated with a marked decrease in the percentage of cells in the G0/G1 phase together with an increase in the number of cells in the S phase (Figure 4A). The addition of the EGCG before PDGF reduced the number of cells in the S phase, and the percentage of cells in the G0/G1 phase was similar to the percentage observed in untreated cells. Thus, the addition of EGCG inhibited PDGF-induced progression of the cell cycle beyond the G1 phase.
Figure 4 EGCG inhibited cell cycle progression beyond G1 phase.
A: PSCs were treated with PDGF-BB (at 25 μg/L) in the absence or presence of EGCG at 25 μmol/L. After 24-h incubation, cells were harvested, and cell cycle analysis was performed by flow cytometry after staining with propidium iodide. Data show the percentage of cells in each phase of the cell cycle in a representative experiment; B: PSCs were treated with PDGF-BB (at 25 μg/L) in the absence or presence of EGCG at the indicated concentrations (μmol/L). After 24-h incubation, cells were harvested, and total cell lysates were prepared. The levels of cyclin D1, p21Waf1, p27Kip1, and GAPDH were determined by Western blotting.
We examined the effects of PDGF and EGCG treatment on cell cycle-related molecules. The level of cyclin D1 expression in serum-starved cells was low, but PDGF increased the expression (Figure 4B). EGCG reduced the PDGF-induced cyclin D1 expression. In contrast, the level of p27Kip1 expression was decr-eased by PDGF treatment, and EGCG increased the expression. p21Waf1 could not be detected in our experimental system.
EGCG inhibited PDGF-induced phosphorylation of PDGF β-receptor, ERK, and Akt
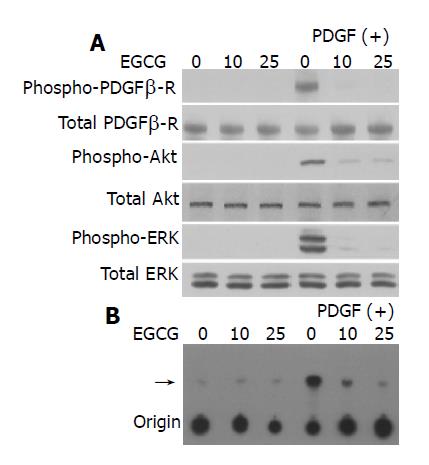
We then attempted to clarify the molecular mechanisms responsible for EGCG’s inhibitory effects on PSCs. PDGF-BB induced tyrosine phosphorylation of the PDGF β-receptor in a time-dependent manner (Figure 5A). EGCG inhibited PDGF-induced phosphorylation of PDGF β-receptor without affecting the protein expression of PDGF β-receptor.
Figure 5 EGCG inhibited phosphorylation of PDGF β-receptor, ERK, and Akt.
PSCs were incubated in the absence or presence of EGCG at the indicated concentrations for 1 h, and then treated with PDGF-BB (at 25 μg/L) for 5 min. A: total cell lysates were prepared, and the total and phosphorylated levels of PDGF β-receptor, Akt, and ERK were determined by Western blotting; B: total cell lysates were prepared and immunoprecipitated with anti-phosphotyrosine antibody. PI3-kinase activity was assessed using PI as a substrate. The arrow denotes the product, PI3-phosphate, resolved by thin layer chromatography.
Previous studies have shown that activation of c-Raf/MAP kinase kinase/ERK and PI3-kinase/Akt pathways plays key roles for PDGF-induced proliferation and migration, respec-tively[11,15]. EGCG inhibited PDGF-BB-induced phosphoryla-tion of ERK and Akt in a dose-dependent manner (Figure 5A). In addition, EGCG inhibited PDGF-induced activation of PI3-kinase (Figure 5B). Thus, EGCG inhibited PDGF-induced tyrosine phosphorylation of PDGF β-receptor and downstream activation of ERK and PI3-kinase/Akt pathways.
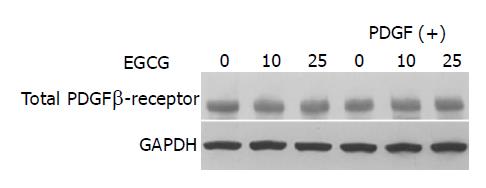
EGCG did not alter the expression of total PDGF β-receptor
It has been shown in hepatic stellate cells that EGCG inhibited cell proliferation by blocking the tyrosine phosphorylation and by reducing the gene expression of PDGF β-receptor[31]. We examined whether EGCG altered the expression of total PDGF β-receptor. EGCG did not alter the expression of total PDGF β-receptor regardless of absence or presence of PDGF (Figure 6).
Figure 6 EGCG did not alter the expression of total PDGF β-receptor.
PSCs were incubated with EGCG at the indicated concentrations in the absence or presence of PDGF-BB (at 25 μg/L). After 24 h, total cell lysates (approximately 100 μg) were prepared, and the levels of total PDGF β-receptor and GAPDH were determined by Western blotting.
DISCUSSION
The present study demonstrated that EGCG at non-cytotoxic concentrations inhibited PDGF-induced proliferation and migration. These inhibitory effects appeared through inhibiting tyrosine phosphorylation of PDGF β-receptor and downstream activation of ERK and PI3-kinase/Akt pathways. This is in agreement with the previous study showing that green tea catechins containing a galloyl group in the third position of the catechin structure interfered with PDGF-BB-induced mitogenic signaling in vascular smooth muscle cells by inhibiting tyrosine phosphorylation of the PDGF β-receptor[22]. The effects of EGCG were not mediated by its non-specific cytotoxicity because the levels of PDGF β-receptor and GAPDH were not decreased. In this study, EGCG was cytotoxic to PSCs above 25 μmol/L. This is relatively low compared to hepatic stellate cells where EGCG was not cytotoxic up to 100 μmol/L[31]. This may reflect differences in experimental conditions, including differences in cell types. Indeed, Chen and Zhang[31] treated hepatic stellate cells with EGCG in the presence of 100 mL/L fetal bovine serum whereas we treated PSCs with EGCG in serum-free medium.
We showed here that EGCG inhibited tyrosine phospho-rylation of PDGF β-receptor. Tyrosine phosphorylation of PDGF β-receptor serves as a critical link between extracellular PDGF stimulation and intracellular signaling[13,14]. It has been shown that EGCG inhibited receptor-type protein tyrosine kinases (epidermal growth factor receptor, PDGF receptor, and fibroblast growth factor receptor) whereas EGCG scarcely inhibited serine- and threonine-specific protein kinases such as protein kinases A and C [32]. Like tyrphostin AG1296 [33], EGCG may induce conformational changes at the ATP-binding site of the PDGF β-receptor, thereby inhibiting its tyrosine phosphorylation. Other types of polyphenolic compounds (namely, genistein and quercetin) inhibit receptor tyrosine kinases via this mechanism[34]. On the other hand, it has been suggested that decreased tyrosine phosphorylation of PDGF receptor might be attributed to decreased binding of PDGF to the receptor. EGCG, as in the case of other flavanols, can form complexes with biologic macromolecule such as lipids, carbohydrates, proteins, and nucleic acids[35]. Suzuki et al[36], demonstrated that PDGF-BB was captured by EGCG immobilized on agarose gel. Weber et al[37], showed that EGCG was incorporated into different cellular components including cell surface membranes, which leads to a trapping of PDGF to non-receptor binding sites and reduced PDGF-BB binding to the respective receptors. Thus, EGCG incorporated into plasma membrane or soluble EGCG might directly interact with PDGF-BB, thereby preventing specific receptor binding.
It should be noted that EGCG here did not affect the total PDGF β-receptor expression. This is in contrast to hepatic stellate cells where EGCG inhibited cell proliferation by blocking the tyrosine phosphorylation and by reducing the gene expression of PDGF β-receptor[31]. In hepatic stellate cells, EGCG blocked the activation of activator protein-1 and nuclear factor κB, which, in turn, resulted in a marked reduction in the promoter activity and gene expression of the PDGF β-receptor[31]. Because EGCG did not alter the amount of PDGF β-receptor, different mechanisms might be involved. We here showed that EGCG inhibited the activation of ERK and PI3-kinase/Akt pathways. Because EGCG inhibited phosphorylation of PDGF β-receptor, it would be logical to assume that the reduction in activity of kinases downstream of PDGF receptor was solely due to the reduction in PDGF receptor activity. However, recent cell-free study showed that EGCG could directly inhibit ERK and Akt activation in response to epidermal growth factor[38]. Thus, activation of ERK and Akt might be inhibited in the cell by dual effects of EGCG: the suppression of incoming PDGF-associated stimulation and direct inhibition of these kinases. Further studies are required to elucidate the complexity of the regulation.
During tissue repair and inflammatory processes in the pancreas, PDGF is secreted by various cells including platelets, mononuclear cells, and activated macrophages[39]. Exposure of PSCs to PDGF in vivo is likely to occur in conditions of pancreatic inflammation characterized by the presence of platelets and activated macrophages[2,10]. PDGF-induced effects on PSCs in vivo may be further aided by the up-regulation of PDGF receptors on the surface of PSCs. In this regard, it should be noted that in a rat model of pancreatic fibrosis, immunostaining for PDGF β-receptor was found to be notably increased in association with areas of fibrosis, and the expression of the PDGF β-receptor, but not of the PDGF α-receptor, was closely associated with desmin staining, suggesting that PSCs expressed the PDGF β-receptor[3]. Codistribution of PDGF with cells expressing its receptor confirms a functional role of PDGF in the development of pancreatic fibrosis.
We showed here that PDGF-BB, which is one of the most potent mitogen for PSCs, induced the expression of cyclin D1 whereas p27Kip1 expression was down-regulated. The effects of PDGF-BB on these cell cycle-related molecules were blocked by EGCG treatment, resulting in the inhibition of cell cycle progression beyond the G1 phase. The D-group cyclin proteins play critical roles in the progression of cells through the G1 phase of the cell cycle[40,41]. It has been reported that overexpression of cyclin D1 in cultured cells shortened G1 phase and caused more rapid entry into S phase[40]. Conversely, microinjection of antisense cyclin D1 oligonucleotide or cyclin D1 antibody arrested the cells at G1 phase[41]. Although regulation of cell cycle progression in PSCs remains largely unknown, our results suggested for the first time that induction of cyclin D1 and decreased p27Kip1 might be a prerequisite for PSC proliferation. Because PSCs were treated with EGCG in the presence of PDGF-BB, down-regulation of cyclin D1 and upregulation of p27Kip1 might merely result from decreased tyrosine phosphorylation of PDGF β-receptor. It should be noted that EGCG by itself inhibited cyclin-dependent kinases 2 and 4 activities but induced p21Waf1 and p27Kip1 expression during growth arrest of human breast carcinoma cells[42].
We have recently shown that 4-hydroxy-2,3-nonenal, an aldehydic end-product of lipid peroxidation, induced type I collagen production in PSCs, suggesting a role of oxidative stress in the regulation of cell functions of PSCs. Although little is known about the effective EGCG concentrations required to modulate PDGF-mediated signaling pathways in vivo, it would be of interest to see whether EGCG inhibits the development of pancreatic fibrosis in vivo. In addition to its inhibitory effects on PDGF-induced cell functions, we have found that EGCG inhibited cytokine-induced production of monocyte chemoattractant protein-1 (Masamune et al, manuscript in preparation). The pharmacokinetics of the green tea polyphenols in humans have been examined in detail[43,44], and the maximum achievable EGCG concentration in vivo is less than the concentrations used in the current study. For example, 1 cup (240 mL) of green tea contains 200 mg of EGCG [43] and a single 200-mg dose of EGCG produces a plasma EGCG concentration of -0.1 μmol/L[44]. However, the consumption of pharmaceutically prepared formulations of green tea polyphenols produces plasma EGCG concent-rations approaching 2 μmol/L[44]. EGCG has been shown to protect against carbon tetrachloride-induced hepatotoxicity in mice[45], and endotoxin-induced lethal shock[46]. Its potent antioxidant capability and long history as beverage without adverse health effects make it a candidate for the treatment of pancreatic fibrosis. Experiments designed to test this hypothesis are under way in our laboratory.