Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3277
Revised: July 6, 2004
Accepted: July 22, 2004
Published online: June 7, 2005
AIM: To investigate the difference of gene expression profiles between Barrett’s esophagus and reflux eso-phagitis induced by gastroduodenoesophageal reflux in rats.
METHODS: Eight-week-old Sprague-Dawley rats were treated esophagoduodenostomy to produce gastroduode-noesophageal reflux, and another group received sham operation as control. Esophageal epithelial tissues were dissected and frozen in liquid nitrogen immediately for pathology 40 wk after surgery. The expression profiles of 4096 genes in reflux esophagitis and Barrett’s esophagus tissues were compared with normal esophageal epithelium by cDNA microarray.
RESULTS: Four hundred and forty-eight genes in Barrett’s esophagus were more than three times different from those in normal esophageal epithelium, including 312 up-regulated and 136 down-regulated genes. Two hundred and thirty-two genes in RE were more than three times different from those in normal esophageal epithelium, 90 up-regulated and 142 down-regulated genes. Compared to reflux esophagitis, there were 214 up-regulated and 142 down-regulated genes in Barrett’s esophagus.
CONCLUSION: Esophageal epithelium exposed excessively to harmful ingredients of duodenal and gastric reflux can develop esophagitis and Barrett’s esophagus gradually. The gene expression level is different between reflux esophagitis and Barrett’s esophagus and the differentially expressed genes might be related to the occurrence and development of Barrett’s esophagus and the promotion or progression in adenocarcinoma.
- Citation: Cheng P, Gong J, Wang T, Jie C, Liu GS, Zhang R. Gene expression in Barrett’s esophagus and reflux esophagitis induced by gastroduodenoesophageal reflux in rats. World J Gastroenterol 2005; 11(21): 3277-3280
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3277
The incidence of esophageal adenocarcinoma has increased considerably in the past few years, which may be related to the increasing incidence of gastroesophageal reflux diseases and Barrett’s esophagus[1,2]. However, the exact mechanism especially the molecular biological mechanism of esophageal adenocarcinoma is unknown. In our study, esophagoduod-enostomies were performed on Sprague-Dawley rats to produce gastroduodenoesophageal reflux according to Miwa[3] and the changes of gene expression profiles between Barrett’s esophagus and reflux esophagitis were investigated by cDNA microarray.
One hundred and twenty healthy Sprague-Dawley rats weighing about 200-250 g were purchased from the Experi-mental Animal Center of Xi’an Jiaotong University. The rats were housed in rat cages at 22-25 °C with free access to standard rat pellet food and water for 40 wk. Rats were treated following the guidelines for the care and use of laboratory animals of National Animal Welfare Committee. Surgical procedures were performed on 90 rats by esophagoduodenostomy to produce gastroduodenoesophageal reflux, and on 30 rats by sham operation[3]. The animals were fed with a standard chow.
All the tissue specimens including esophagitis, Barrett’s esophagus were taken from animals 40 wk after esophagoduodenostomy, by which gastroduodenoesophageal reflux animal models were produced. Normal esophageal epithelium of sham operation at the same anatomical site served as normal control. For each sample, the inner part of each sample was cut and frozen in liquid nitrogen immediately after surgical resection, and outer marginal part was used for histopathological examination to ensure that all the frozen tissue specimens their corresponding histological appearance.
Four thousand and ninety-six target cDNA clones were used in cDNA microarray (United Gene Ltd.). These genes were amplified with PCR using universal primers and then purified with standard method. The quality of PCR was monitored by agarose gel electrophoresis. The obtained genes were dissolved in 3×SSC spotting solution and then spotted on silylated slides (TeleChem, Inc.) by Cartesian 7500 spotting robotics (Cartesian, Inc.). Each target gene was dotted twice. After spotting, the slides were hydrated (2 h) and dried (0.5 h, room temperature). The samples were cross-linked with UV light and treated with 0.2% SDS, H2O and 0.2% NaNBH4 for 10 min. Then the slides were dried in cold condition and ready for use.
Total sample RNA was extracted by single step method[5]. Briefly, after taken out from liquid nitrogen specimens were ground completely into tiny powder while liquid nitrogen was added in ceramic mortar and then the powder was homogenized in D solution plus 1% mercaptoethanol. After centrifugation, the supernatant was extracted with phenol: chloroform (1:1) and NaAC and acidic phenol: chloroform (5:1). The aqueous phase was precipitated by an equal volume of isopropanol and centrifuged. The precipitates were dissolved with Millie-Q H2O. After further purification by LiCl precipitating method was obtained RNA, good in quality with UV analysis and electrophoresis. The mRNAs were isolated and purified with Oligotex mRNA Midi kit (Qiagen, Inc.). The fluorescent-labeled cDNA probe was prepared through retro-transcription, in reference to the method of Schena[4]. The probes from normal epithelium were labeled with Cy3-dUTP, while those from Barrett’s esophagus and esophagitis epithelium with Cy5-dUTP. The probes were mixed (Cy3-dUTP control+Cy5-dUTP Barrett’s esophagus epithelium and Cy3-dUTP control+Cy5-dUTP esophagitis epithelium) and precipitated by ethanol, and then resolved in 20 mL hybridization solution (5×SSC +0.2% SDS).
Probes and the chip were denatured in 95 °C bath for 5 min, and then the probes were added on to the chip. They were hybridized in a sealed chamber at 60 °C for 15-17 h, washed in turns with solutions of 2×SSC+0.2% SDS, 0.1×SSC+0.2% SDS and 0.1% SSC for 10 min each, and then dried at room temperature.
The chip was read by Scan Array 3000 Scanner (General Scanning Inc.). The overall intensities of Cy3 and Cy5 were normalized and corrected by a coefficient according to the ratios of the located 40 housekeeping genes. Cy3 was normalized as Cy3*. The acquired image was further analyzed by Gene Pix Pro 3.0 software with digital computer to obtain the intensities of fluorescent signals and the Cy5/Cy3* ratio. The data was an average of the two repeated spots. The differentially expressed genes were defined as: (1) Absolute value of the Cy5/Cy3* natural logarithm was more than 1.10 (the variation of gene expression was more than three folds). (2) Either Cy3 or Cy5 signal value was required for more than 800, or both signal values were more than 200. (3) PCR results were satisfactory.
Total sample RNA of normal esophageal epithelium, reflux esophagitis and Barrett’s esophagus was extracted and the D260/D280 was between 2 and 2.6, indicating pure mRNA was acquired.
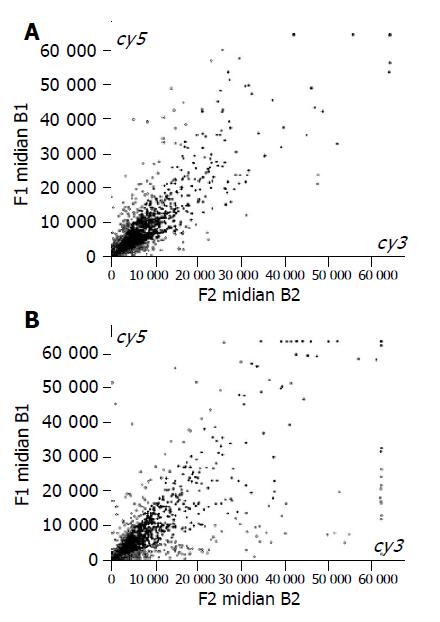
The scatter plot that was plotted with Cy3 and Cy5 fluorescent signal values displayed quite a dispersed pattern in distribution. Most of the spots gathered around a 45 angle diagonal line in which the blue spots represented the area where the signal intensities varied between 0.33 to 3 folds compared with that of the control. Some yellow spots distributed beyond or far from 45 angle diagonal line indicating the existence of abnormal gene expression in Barrett’s esophagus or esophagitis epithelium and their signal intensities were three times more or less than that of the control (Figure 1A and 1B).
In esophagitis epithelia 232 genes showed expression variations more than three times from the control, the up and down-regulated genes numbered 90 and 142 respectively. In Barrett’s esophagus epithelia 448 genes exhibited expression variations more than three times than the control, the up and down-regulated genes were 312 and 136 respectively. Difference of gene expression between esophagitis epithelia and Barrett’s esophagus was acquired by subtracting genes in common from the total genes expressed differently of esophagitis epithelia and Barrett’s esophagus. Three hundred genes expressed differentially in Barrett’s esophagus compared to those in esophagitis epithelia, and the up and down-regulated genes were 214 and 86 respectively. Some genes are listed in Tables 1 and 2. These genes might be divided into 12 groups (Table 3) according to their functions.
| Up-regulated gene |
| Glutathione synthetase |
| S100 calcium binding protein A9 |
| alpha-fibrinogen |
| alpha-1-protease inhibitor |
| Lyn protein non-receptor kinase |
| Polymeric immunoglobulin receptor |
| Prostaglandin D2 synthase 2 |
| Serine protease inhibitor |
| Kangai 1 |
| Hemopexin |
| Fibrinogen |
| DOC-2 p82 isoform |
| Leukemia-associated cytosolic phosphoprotein |
| Junctional adhesion molecule 1 |
| Interleukin 1 beta |
| Cell adhesion regulator |
| Down-regulated gene |
| BCL2/adenovirus E1B 19-ku-interacting protein-3 |
| Transforming growth factor b stimulated clone-22 |
| Tissue inhibitor of metalloproteinase-3 |
| Phosphoglyceromutase |
| CD36 antigen |
| Calsequestrin 2 |
| Transgelin |
| Mitochondrial adenine nucleotide translocator |
| Glucocorticoid-induced leucine zipper |
| Extracellular matrix protein 2 |
| Protein tyrosine phosphatase |
| Functional classification | n | Up-regulated | Down-regulated |
| Oncogenes and tumor suppressor genes | 14 | 12 | 2 |
| Ion channel and transporters | 30 | 16 | 14 |
| Cell cycle proteins | 21 | 18 | 3 |
| Extra-pressure reaction proteins | 20 | 18 | 2 |
| Cell regulatory proteins | 13 | 9 | 4 |
| Cell apoptosis related proteins | 14 | 10 | 4 |
| DNA synthesis, repair and recombinant factors | 25 | 17 | 8 |
| DNA binding, transcription factors | 30 | 22 | 8 |
| Cell receptors | 33 | 27 | 8 |
| Immunity-related proteins | 29 | 17 | 12 |
| Cell signal transduction proteins | 40 | 27 | 13 |
| Metabolism related proteins | 31 | 21 | 10 |
| Total | 300 | 214 | 86 |
Some researchers confirmed that Barrett’s esophagus is related to esophageal adenocarcinoma closely. The annual rate of Barrett’s esophagus developing into esophageal adenocarcinoma increased by 0.5% and the former was regarded as the precancerous lesion of the latter[2]. However, in those reports adenocarcinoma of the gastroesophageal junction was thought as esophageal adenocarcinoma, which mixed up the tissue origin of esophageal adenocarcinoma. Some clinical epidemiological data on cardiac and esophageal adenocarcinoma indicated that the two diseases were similar in respects of age, sex, clinical, and pathological characteristics so that the origin of the two diseases was thought as the same tissue[5]. And both the diseases were known as adenoc-arcinoma of gastroesophageal junction[6].
Whether Barrett’s esophagus is the precancerous disease of esophageal adenocarcinoma is further studied by Miwa’s[3] research, which suggested that esophageal adenocarcinoma arose from Barrett’s esophagus, which is induced by gastro-duodeno-esophageal reflux. Single gene research on esophageal adenocarcinogenesis has been done[7]. The carcinogenesis is a process involving multiple steps and factors and caused by abnormal expression of tumor-associated genes or inactivation of tumor suppression genes or both. Therefore clarifying the gene expression differences between malignant, precancerous and normal tissues is the key procedure for the cancer control study. It is generally accepted that although the number of genes with mutation is limited in a cancer, a great number of genes in related pathways may be affected at the expression level, and this aberrant gene transcriptional expression network should be essential in the initiation/maintenance of the malignant phenotype. With the advances of molecular biological techniques, gene chip has been used to detect gene expression difference in various specimens by parallel analysis on a large scale[8]. In our research, animal model of gastro-duodeno-esophageal reflux was made with reference to Miwa[3] and the changes of gene expression profiles between Barrett’s esophagus and reflux esophagitis were investigated by cDNA microarray.
Among the up-regulated genes, cell adhesion regulator 1, leukemia-associated cytosolic phosphoprotein, glutathione synthetase, kangai 1, junctional adhesion molecule 1 and S100 calcium-binding protein A9 were all related to the development of tumor. The transmembrane protein, junctional adhesion molecule 1, causes the protein CagA into gastric epithelial cells and associates with peptic ulcer disease and carcinoma[9]. It might be participating in the epithelium in jury by harmful ingredients of duodenal and gastric reflux. Leukemia-associated cytosolic phosphoprotein is supposed to play a role in regulation of cell proliferation or the proliferation-differentiation switch and expressed vigorously in all but one of 85 diverse tumor cell lines and primary human malignant tumors examined[10]. Its up-regulated expression indicated over proliferation of Barrett’s esophagus compared with reflux esophagitis. Apoptotic signaling after genotoxic exposure can be inhibited by the antioxidant activity of glutathione[11]. The up-regulation of cell adhesion regulator is associated with the progression of colorectal tumors, while that of kangai 1 seems to be involved in the early stage[12]. Up-regulation of the two genes in Barrett’s esophagus illuminated Barrett’s esophagus might be inclined to developing into tumor. Up-regulated expression of the hemopexin is involved in enhanced neoplastic cell invasion and migration[13]. S100 calcium-binding protein A9 gene expression has been detected in cultured human adenocarcinoma cells derived from various organs[14].
Among the down-regulated genes, extracellular matrix protein 2, protein tyrosine phosphatase 2E, tissue inhibitor of metalloproteinase 3, CD36 antigen, transforming growth factor β stimulated clone-22, and BCL2/adenovirus E1B 19-ku-interacting protein-3 were involved in suppressing tumor, indicating the ability of esophagus against tumor decreased with normal esophageal epithelium developing into Barrett’s esophagus by over exposure to contents of gastric and duodenal reflux. Extracellular matrix protein 2 mRNA expression levels decreased in many metastasis specimens, it might be related to invasion and migration of tumor[15]. Protein tyrosine phosphatase was associated with the cell signaling control, energy metabolism, proliferation and promotion of MHC-I antigen expression, mediated by numerous hormones (such as epidermal growth factor, insulin, insulin-like growth factor 1)[16]. The down-regulated PTPase would decrease the antigen expression on the cell surface, and result in malignant cells escaping from the immune surveillance. In the present study, the enzyme was also down-regulated in Barrett’s esophagus. Tissue inhibitor of metalloproteinase-3 inhibits the activity of metalloprotease and the latter is an important protein associated with tumor invasion and metastasis. In general, the former is down-regulated and the latter up-regulated in tumor tissue[17]. CD36 antigen is the cellular receptor for thrombospondin-1 on microvascular endothelium and is necessary for its anti-angiogenic activity with down-regulation in many tumor tissues. Transforming growth factor beta-stimulated clone-22 mediated inhibition of apoptosis and was down-regulated in over proliferated tissues. BCL2/adenovirus E1B 19-ku-interacting protein-3 had growth inhibitory effect on cancer cells and was down-regulated in many tumors.
Many up-regulated or down-regulated genes in Barrett’s esophagus compared to reflux esophagitis indicated that a great number of genes in related pathways might affect the carcinogenesis of Barrett’s esophagus. The application of gene chip technique is a revolution of research method in life science. Our experiment illustrated that the detection of gene expression difference between Barrett’s esophagus and reflux esophagitis by gene chip might disclose the molecular mechanism of the onset, promotion and progression of Barrett’s esophagus into esophageal adenocarcinoma and provide a new direction for diagnosis, therapy and prevention of esophageal carcinoma.
Science Editor Zhu LH Language Editor Elsevier HK
| 1. | O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037-2042. [PubMed] |
| 2. | Geboes K. Barrett's esophagus: the metaplasia-dysplasia-carcinoma sequence: morphological aspects. Acta Gastroenterol Belg. 2000;63:13-17. [PubMed] |
| 3. | Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614-10619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 958] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer. 2000;88:2520-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Byrne JP, Mathers JM, Parry JM, Attwood SE, Bancewicz J, Woodman CB. Site distribution of oesophagogastric cancer. J Clin Pathol. 2002;55:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bektas N, Donner A, Wirtz C, Heep H, Gabbert HE, Sarbia M. Allelic loss involving the tumor suppressor genes APC and MCC and expression of the APC protein in the development of dysplasia and carcinoma in Barrett esophagus. Am J Clin Pathol. 2000;114:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Huang GS, Yang SM, Hong MY, Yang PC, Liu YC. Differential gene expression of livers from ApoE deficient mice. Life Sci. 2000;68:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 582] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 10. | Ghosh PK, Anderson J, Cohen N, Takeshita K, Atweh GF, Lebowitz P. Expression of the leukemia-associated gene, p18, in normal and malignant tissues; inactivation of expression in a patient with cleaved B cell lymphoma/leukemia. Oncogene. 1993;8:2869-2872. [PubMed] |
| 11. | Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312-318. [PubMed] |
| 12. | Mikami T, Ookawa K, Shimoyama T, Fukuda S, Saito H, Munakata A. KAI1, CAR, and Smad4 expression in the progression of colorectal tumor. J Gastroenterol. 2001;36:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Takino T, Miyamori H, Watanabe Y, Yoshioka K, Seiki M, Sato H. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Cancer Res. 2004;64:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Arai K, Teratani T, Nozawa R, Yamada T. Immunohistochemical investigation of S100A9 expression in pulmonary adenocarcinoma: S100A9 expression is associated with tumor differentiation. Oncol Rep. 2001;8:591-596. [PubMed] |
| 15. | Hao X, Sun B, Hu L, Lähdesmäki H, Dunmire V, Feng Y, Zhang SW, Wang H, Wu C, Wang H. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004;100:1110-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Yamamoto Y, Irie K, Asada M, Mino A, Mandai K, Takai Y. Direct binding of the human homologue of the Drosophila disc large tumor suppressor gene to seven-pass transmembrane proteins, tumor endothelial marker 5 (TEM5), and a novel TEM5-like protein. Oncogene. 2004;23:3889-3897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Karan D, Lin FC, Bryan M, Ringel J, Moniaux N, Lin MF, Batra SK. Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int J Oncol. 2003;23:1365-1371. [PubMed] |