Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3245
Revised: August 14, 2004
Accepted: September 30, 2004
Published online: June 7, 2005
AIM: To assess the expression of Ki67 as prognosticator in rectal/recto sigmoid cancer.
METHODS: Samples from 146 patients with rectal and recto sigmoid cancer were studied for expression of Ki67 and its prognostic significance in comparison with clinico-pathological predictors of survival. Formalin-fixed, paraffin-embedded tissues from 6 (4.1%) patients with T1, 26 (17.8%) with T2, 94 (64.4%) with T3, and 20 (13.7%) with T4 tumors were studied. Ki67 expression was determined immunohistochemically. Samples were divided according to mean value into high (>40%) and low (≤40%) expression. Areas of extensive proliferation (>50%) were defined as ‘hot spot’ areas.
RESULTS: Hot spot areas were present in samples regardless of histopathological grade. Lower TNM and Dukes stage and higher expression of Ki67 and presence of Ki67 hot spot areas in histopathological samples were associated with better survival, whereas no association was observed with histopathological grade (P = 0.78). In Cox multivariate regression analysis, significant prognostic factors were Dukes stage (P<0.001), presence of lymph node metastases (P = 0.015), age (P = 0.035) and presence of Ki67 hot spot areas (P = 0.044).
CONCLUSION: Proliferative activity as measured by Ki67 in rectal cancer is associated with survival improvement compared with patients with low Ki67. Areas of prognostically significant increased proliferation were found independently of histopathological tumor grade.
- Citation: Salminen E, Palmu S, Vahlberg T, Roberts PJ, Söderström KO. Increased proliferation activity measured by immunoreactive Ki67 is associated with survival improvement in rectal/recto sigmoid cancer. World J Gastroenterol 2005; 11(21): 3245-3249
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3245.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3245
Colorectal cancer is the third most common cancer in the Western world and constitutes the second leading cause of cancer deaths[1]. Its incidence has been increasing steadily during recent years. The tumor biology of rectal adenocarcinoma is different from that of upper colon cancers. Rectal cancer is characterized by particular biological and genetic features and clinical behavior[2].
Ki67 is a proliferation antigen which is expressed during all phases of the cell cycle except for the resting of cells in G0[3]. Ki67 can be detected in proliferating cells in both frozen and paraffin-embedded tissue[4]. This specific immunoreactivity is associated with tumor proliferation[3] and the Ki67 labeling index has prognostic significance in various types of carcinomas. In many cancers, including cancer of the breast, malignant lymphomas and astrocytomas, a high proliferation rate expressed by Ki67 antigen has been shown to have prognostic significance[5-8].
In colorectal cancer, contradictory results have been reported on associations of Ki67 with prognosis and survival. Palmqvist and collaborators[9] reported colorectal tumors with a low proliferation index in Dukes B tumors to be associated with survival impairment compared to those with high values. Willett and associates[10] measured proliferative activity after preoperative irradiation in rectal cancer. They observed that patients whose tumors showed higher proliferative activity after radiation treatment survived better compared to those with tumors of lower proliferation. In contrast, Kimura and collaborators[11] reported poor prognosis in colorectal tumors with a high proliferative index. The reasons for these discrepancies have remained open.
As reports on proliferative activity and survival in rectal cancer are scant and the association of low proliferation with poor survival is limited to Dukes B tumors, we undertook to study the association of Ki67 in all stages of rectal tumors to clarify its prognostic role for patients with rectal or recto sigmoid cancers.
One hundred and forty-six patients with recto sigmoid and rectal cancer who were treated for primary (135 patients, 92%) or recurrent (11 patients, 8%) cancer at the University Hospital in Turku in 1992-2002 were included in this study. Criteria for inclusion were as follows: histologically proven recto sigmoid or rectal carcinoma with the upper lever below 24 cm, no preoperative chemo- or radiotherapy. Twenty-one tumors (14%) were located in the rectosigma (17-24 cm from anus) and 125 (86%) in the rectum (<17 cm). All patients had been operated on for cancer, either with anterior resection or rectum amputation. Most patients received postoperative adjuvant radiotherapy given with a linear accelerator using conventional fractionation and dose planning to a mean dose of 50.4 Gy to the tumor area.
TNM classification (UICC 1997)[12] and Dukes staging[13] were used for staging. The samples were re-evaluated by an experienced pathologist who graded the tumors from I to III, depending on the degree of anaplasia and nuclear size according to the World Health Organization[14].
From paraffin-embedded blocks, 5-μm-thick sections were cut, deparaffinized with xylene and rehydrated through a graded series of alcohol. For antigen retrieval, the samples were boiled for 10 min in a microwave oven in 10 mmol/L sodium citrate buffer (pH 6.0). An automated processor (TechMate 500, DAKO) was used for immunohistochemical staining. Steps were performed in the immunostainer using the avidin-biotin-peroxidase staining method. Mouse monoclonal Ki67 antibody MIB-1 antibody (DAKO, Denmark) was diluted 1:100 and applied for 27 min. The percentage of immunoreactive nuclei of Ki67 was counted for each tumor slide. The reaction was considered positive when 10% or more of the cancer cells showed staining. The cut-off point was chosen to indicate cases with clear positive staining. As a negative control the primary antibody was omitted from each staining. No significant background staining was detectable.
The expression of Ki67 was evaluated by two observers (SP and KOS) independently and they were blinded to the clinical data at this stage. A consensus was sought for differences in opinion. The total expression of Ki67 was evaluated in the whole sample. The amount of positively stained cells was counted in different areas and the total expression then estimated in percentages. Areas of extensive proliferation (>50%) were defined as ‘hot spot’ areas. A hot spot area was described as an area seen with a 10×objective with the highest number of Ki67 positively stained cells.
Univariate associations between variables were evaluated by χ2 test or Fisher’s exact test. The Kaplan-Meier survival curves were compared using log-rank test. Univariate and multivariate associations between risk factors and survival were analyzed with Cox proportional hazard models. P values less than 0.05 were considered statistically significant. Statistical computations were made with SAS System for Windows, release 8.02.
Patient characteristics are presented in Table 1. The mean age of the patients was 66 years (range 36-86 years). Six (4%) presented with T1 tumors, 26 (18%) with T2, 94 (64%) with T3 and 20 (14%) with T4 tumors, corresponding to Dukes A in 27 patients (18%), with B in 86 patients (57%), with C in 25 patients (17%) and with D in 12 patients (8%). Histological sample re-evaluation for grade according to the WHO classification indicated 24 tumors (16%) of grade 1, 89 (61%) of grade 2 and 33 (23%) of grade 3. Based on tumor growth to surgical margins or the presence of lymph node metastases, radiotherapy was given to 91 (62%) patients at a median dose of 50.4 Gy (range 48-67 Gy). One patient received only a dose of 25 Gy.
| Number of patients (%) | |
| Total number of eligible patients | 146 (100) |
| Sex | |
| Male | 87 (60) |
| Female | 59 (40) |
| Age (yr) | |
| Mean | 66 |
| Range | 3686 |
| Performance status (WHO) | |
| 0 | 11 (15) |
| 1 | 53 (72) |
| 2 | 10 (14) |
| Tumor histology | |
| Grade 1 | 24 (16) |
| Grade 2 | 89 (61) |
| Grade 3 | 33 (23) |
| Tumor localization | |
| Rectum | 125 (86) |
| Recto sigmoid | 21 (14) |
| T stage | |
| T1 | 6 (4) |
| T2 | 26 (18) |
| T3 | 94 (64) |
| T4 | 20 (14) |
| Dukes | |
| A | 26 (18) |
| B | 85 (58) |
| C | 25 (17) |
| D | 10 (7) |
| N stage | |
| 0 | 98 (67) |
| 1 | 26 (18) |
| 2 | 22 (15) |
| Primary tumor | 135 (93) |
| Recurrent tumor | 11 (8) |
| Radiotherapy | |
| Given to N patients (%) | 91 (62) |
| Median dose (range) Gy1 | 50 (4867) |
Hot spot areas were found in tumor samples regardless of the degree of average Ki67 proliferation, similarly among tumors with overall low, higher and highest degree of proliferation, e.g., under and above the cut-off level of 40% or 50%, which represented the median values in samples. The significance of associations of Ki67 proliferation with clinico-pathological factors was tested by χ2 test. The associations with N-stage, Dukes and hot spot areas were significant (P = 0.020, P = 0.012 and P<0.001, respectively) (Table 2). No statistically significant associations were observed with sex, age (cut point 65 years), grade, T-stage, radiotherapy or primary vs. recurrent tumor.
| Ki67 expression | P | ||
| ≤40 | >40 | ||
| n (%) | n (%) | ||
| N-stage | 0.020 | ||
| 0 | 50 (63) | 48 (73) | |
| 1 | 12 (15) | 14 (21) | |
| 2 | 18 (23) | 4 (6) | |
| Dukes | 0.012 | ||
| 1 | 9 (11) | 17 (26) | |
| 2 | 53 (66) | 32 (48) | |
| 3 | 10 (13) | 15 (23) | |
| 4 | 8 (10) | 2 (3) | |
| Hot spot | <0.001 | ||
| ≤50 | 65 (81) | 10 (15) | |
| >50 | 15 (19) | 56 (85) | |
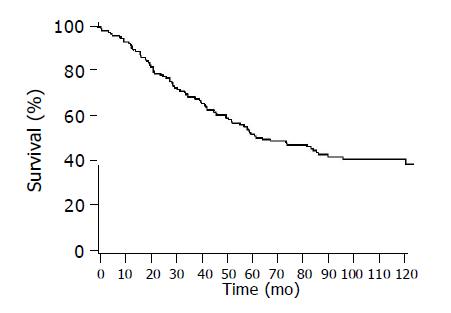
The follow-up time of the surviving patients estimated from the time of operation ranged from 42 to 156 mo, with a median of 99 mo (Figure 1). The median survival time of the patients was 72 mo.
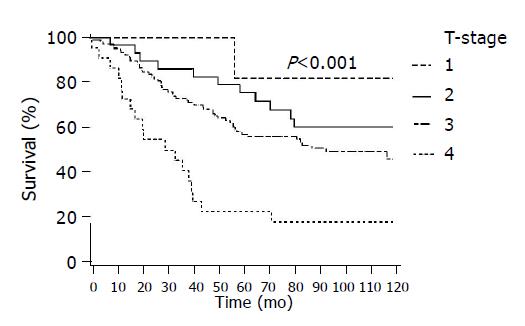
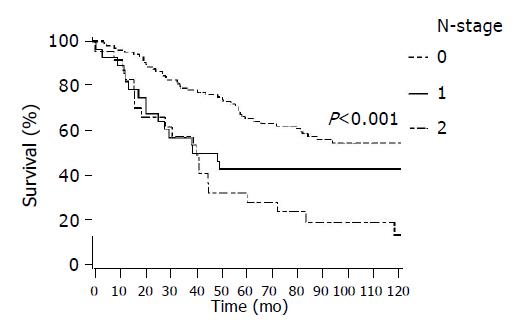
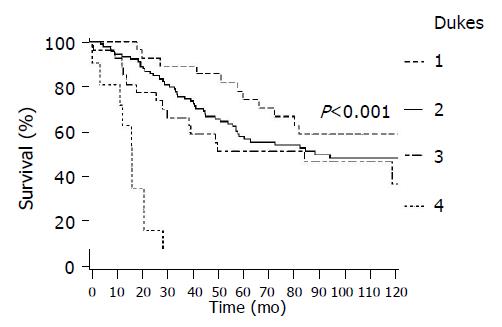
In Figure 2 survival by T-stage is presented showing a decrease in survival with increasing T-stage (P<0.001), and similarly in Figure 3 by Dukes stage (P<0.001) and Figure 4 by lymph node status (P<0.001). In all of these figures lower presentation indicated better survival.
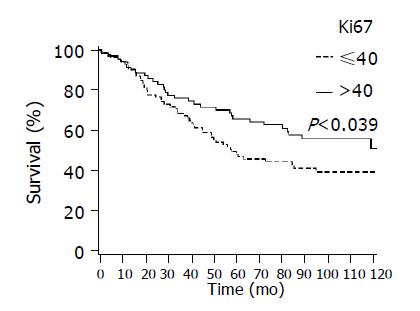
Figure 5 shows better survival among patients with higher proliferation Ki67 compared to those with lower values (P = 0.039).
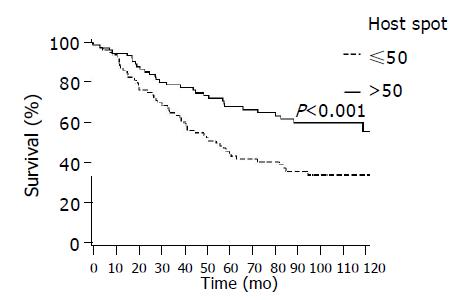
Figure 6 shows survival by presence or absence of Ki67 hot spot areas, with significantly better survival seen among patients with higher hot spot presentation (P = 0.001).
No statistically significant association with survival was observed by sex (P = 0.56), age with a cut-off point at 65 years (P = 0.05), histopathological grade (P = 0.78) or tumor localization (P = 0.42).
Cox’s proportional hazard model was used to quantify the independent contribution of clinical factors and Ki67 to survival. The results are presented in Table 3. The significant prognosticators in the multivariate model were Dukes stage (P<0.001), N-stage (P = 0.015), age (P = 0.035) and presence of Ki67 hot spot areas (P = 0.044).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age (>65 vs <65 yr) | 1.54 (0.99-2.39) | 0.056 | 1.62 (1.04-2.52) | 0.035 |
| T-stage (3+4 vs 1+2) | 2.24 (1.21-4.23) | 0.010 | 1.61 (0.86-3.03) | 0.139 |
| N-stage (1+2+3 vs 0) | 2.42 (1.57-3.74) | <0.001 | 1.82 (1.12-2.94) | 0.015 |
| Dukes (4 vs 1+2+3) | 11.42 (5.342-4.43) | <0.001 | 6.32 (2.781-4.39) | <0.001 |
| Ki67 (>40 vs ≤40) | 0.63 (0.41-0.98) | 0.040 | 1.26 (0.67-2.39) | 0.469 |
| Ki67 Hot spot (>50 vs ≤50) | 0.48 (0.31-0.75) | 0.001 | 0.51 (0.27-0.98) | 0.044 |
Cancer of the rectum and recto sigmoid area belongs to the commonest cancers in the Western world, with relatively poor prognosis. They show a common tendency to both local recurrences and distant metastases[15]. The constant increase in the incidence of these cancers both among men and women in recent years makes characterization of tumor types and identification of new prognosticators important. Histopathological characterization differs from that in other tumors and contradictory observations on the prognostic role of Ki67 proliferative activity have been reported[9,11]. The results of this current study indicate that higher expression of the proliferative antigen indicates better survival in rectal and recto sigmoid cancer.
The survival of patients with colorectal cancer depends on the extent of the tumor and metastatic spread at presentation; patients with advanced stage and/or metastatic lymph nodes at presentation have poor prognosis compared to those with locally limited tumors, as also reflected in the current results. Adjuvant treatment improves disease control in locally advanced carcinoma, and it has been the standard treatment over the period of this study. The overall survival of 60% at 5 years and 45% at 10 years observed in the present series is within the range for this patient group[16]. When used as a continuous variable, age was not associated with survival, though with the cut-off at 65 years the younger patients showed significantly better survival when compared to older ones. T-stage and Dukes classification are known to be valuable prognostic tools in the characterization of colorectal tumors. They describe the extent of growth and correlate with survival similarly, as seen in the present results. The spread of cancer to lymph nodes is often an independent sign of poorer prognosis, regardless of other tumor characteristics.
Pre-treatment evaluation of tumors is difficult due to their location. Contrary to what is seen in many tumors, in colorectal cancer histopathological grade alone does not characterize tumor prognosis. In tumors at this location, the presentation of tumors of the same stage can be heterogeneous when measured by proliferation. In the present study, Ki67 acted independently of histopathological grade and as independent prognostic factor produced more prognostic information for survival than grade. Histological grade was not an independent prognosticator, results being similar to those of Palmqvist and coworkers[9].
Possible explanations for deviation/discrepancies from what is seen with other tumors in the role of Ki67 as a cancer prognosticator have been suggested, including preoperative procedures and tumor growth type. In rectal tumor samples, preoperative colon cleaning with laxatives and enemas has been shown to induce mucosal proliferative activity[17]. Preoperative cleaning was used routinely during the time of operation of the patients in the present series. Another explanation suggests that ulcerative processes may increase proliferation at the luminal border[9], resulting in higher Ki67 expression. This could explain the connection among larger tumor size, advanced stage, and higher proliferative activity without impairment to survival. It has also been argued that the true proliferation rate of cancer cannot be measured immunohistochemically, because it is a function of both growth fraction and the time required for completion of the cell cycle[18-20]. Duchrow and colleagues[20] have presented a comparison of expression of pKi67 mRNA and protein in colorectal cancer and shown that tumors with a high pKi67 protein level but low mRNA expression may proliferate more slowly than estimated if only their Ki67 staining index is taken into account, which may explain the patients’ improved outcome. They suggest that massive expression of pKi67 in tumor cells possibly induces growth arrest and contributes to its own stabilization[20]. A role for Ki67 determination as a tool in selecting and monitoring colorectal cancer patients for radiotherapy has been suggested[21]. However, a recent study has failed to show an association between Ki67 and response in preoperative chemo-radiotherapy in rectal cancer[22].
The prognostic value of Ki67 determination in colorectal cancer may vary depending on how the sample is taken[18]. Kimura and coworkers[11] observed that samples taken for Ki67 determination at the site of the deepest tumor showed poor prognosis for patients with high Ki67 values. Systematic heterogeneity in colorectal samples with higher proliferative activity at the luminal border compared with the invasive margin has been observed and reported[9]. We here standardized the samples by systematic evaluation of hot spot areas as the parameter of observation to overcome heterogeneity in the samples. Hot spot areas could be observed throughout the study samples independently of histopathological grade, which in most other tumors is associated with the degree of proliferation activity. A significant association between hot spot presentation of Ki67 and improved survival was recorded in both uni- and multivariate analysis, and provides new insight regarding the biological behavior of these tumors.
The present finding of an association between high proliferation rate and survival improvement is concordant to earlier observations noted in more highly selected patient series[9,10]. Palmqvist and coworkers[9] showed that a lower tumor proliferation rate at the invasive margin was associated with poor prognosis in Dukes B colorectal cancer. To our knowledge the present is the first report on Ki67 as a prognosticator in all Dukes stages in rectal cancer.
Tumors with high proliferative activity are known to be most responsive to radiotherapy. Willett and collaborators[10] showed that radiation eradicates preferentially rapidly dividing cells in rectal cancer, whereas populations with slow proliferation show greater radioresistance. Their conclusion was that more than minimal proliferative activity in T3 and T4 tumors indicates a survival improvement with radiotherapy. Thus, although survival is reduced with the increase in T or D stage in general, within each tumor stage those with higher proliferation had better disease-free survival and response to conventional radiotherapy/chemotherapy. No association was shown in a recent microarray study between Ki67 expression and distant metastases in rectal cancer[23]. Tumors with lower proliferative activity may need different adjuvant approaches or sensitizers, which could make them more responsive to treatment.
In conclusion, determination of immunohistochemical Ki67 hot spot areas, which act as independent prognosticators, contributes to the prognostic evaluation of patients with rectosigmoid and rectal cancer and in considering them for postsurgical treatment.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | WHO: The World Health Report. Geneva, World Health Organization, 1997. . |
| 2. | Kapiteijn E, Liefers GJ, Los LC, Kranenbarg EK, Hermans J, Tollenaar RA, Moriya Y, van de Velde CJ, van Krieken JH. Mechanisms of oncogenesis in colon versus rectal cancer. J Pathol. 2001;195:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Isola JJ, Helin HJ, Helle MJ, Kallioniemi OP. Evaluation of cell proliferation in breast carcinoma. Comparison of Ki-67 immunohistochemical study, DNA flow cytometric analysis, and mitotic count. Cancer. 1990;65:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Garrido MC, Cordell JL, Becker MH, Key G, Gerdes J, Jones M, Gatter KC, Mason DY. Monoclonal antibody JC1: new reagent for studying cell proliferation. J Clin Pathol. 1992;45:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Kirla R, Salminen E, Huhtala S, Nuutinen J, Talve L, Haapasalo H, Kalimo H. Prognostic value of the expression of tumor suppressor genes p53, p21, p16 and prb, and Ki-67 labelling in high grade astrocytomas treated with radiotherapy. J Neurooncol. 2000;46:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME, Venkatesan V, Lawton CA, Roach M, Shipley WU. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Bui MH, Visapaa H, Seligson D, Kim H, Han KR, Huang Y, Horvath S, Stanbridge EJ, Palotie A, Figlin RA. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Tisell LE, Oden A, Muth A, Altiparmak G, Mõlne J, Ahlman H, Nilsson O. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer. 2003;89:2093-2097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Palmqvist R, Sellberg P, Oberg A, Tavelin B, Rutegård JN, Stenling R. Low tumour cell proliferation at the invasive margin is associated with a poor prognosis in Dukes' stage B colorectal cancers. Br J Cancer. 1999;79:577-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Willett CG, Warland G, Hagan MP, Daly WJ, Coen J, Shellito PC, Compton CC. Tumor proliferation in rectal cancer following preoperative irradiation. J Clin Oncol. 1995;13:1417-1424. [PubMed] |
| 11. | Kimura T, Tanaka S, Haruma K, Sumii K, Kajiyama G, Shimamoto F, Kohno N. Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int J Oncol. 2000;16:55-64. [PubMed] |
| 12. | UICC International Union Against cancer: TNM Classification of malignant tumours. 5th edition, WHO, Geneva, 1997. . |
| 13. | Beahrs OH. Colorectal cancer staging as a prognostic feature. Cancer. 1982;50:2615-2617. [PubMed] |
| 14. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 15. | Köckerling F, Reymond MA, Altendorf-Hofmann A, Dworak O, Hohenberger W. Influence of surgery on metachronous distant metastases and survival in rectal cancer. J Clin Oncol. 1998;16:324-329. [PubMed] |
| 16. | Påhlman L. Rectal carcinoma, type of surgery and the role of adjuvant radiation therapy. Ann Chir Gynaecol. 1993;82:153-158. [PubMed] |
| 17. | Lehy T, Abitbol JL, Mignon M. Influence of rectal preparation by enema on cell proliferation in the normal rectal mucosa in man. Gastroenterol Clin Biol. 1984;8:216-221. [PubMed] |
| 18. | Jansson A, Sun XF. Ki-67 expression in relation to clinicopathological variables and prognosis in colorectal adenocarcinomas. APMIS. 1997;105:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | van Oijen MG, Medema RH, Slootweg PJ, Rijksen G. Positivity of the proliferation marker Ki-67 in noncycling cells. Am J Clin Pathol. 1998;110:24-31. [PubMed] |
| 20. | Duchrow M, Ziemann T, Windhövel U, Bruch HP, Broll R. Colorectal carcinomas with high MIB-1 labelling indices but low pKi67 mRNA levels correlate with better prognostic outcome. Histopathology. 2003;42:566-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Porschen R, Lohe B, Hengels KJ, Borchard F. Assessment of cell proliferation in colorectal carcinomas using the monoclonal antibody Ki-67. Correlation with pathohistologic criteria and influence of irradiation. Cancer. 1989;64:2501-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Reerink O, Karrenbeld A, Plukker JT, Verschueren RC, Szabó BG, Sluiter WJ, Hospers GA, Mulder NH. Molecular prognostic factors in locally irresectable rectal cancer treated preoperatively by chemo-radiotherapy. Anticancer Res. 2004;24:1217-1221. [PubMed] |
| 23. | Fernebro E, Bendahl PO, Dictor M, Persson A, Fernö M, Nilbert M. Immunohistochemical patterns in rectal cancer: application of tissue microarray with prognostic correlations. Int J Cancer. 2004;111:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |