Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. May 21, 2005; 11(19): 2858-2863
Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2858
Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2858
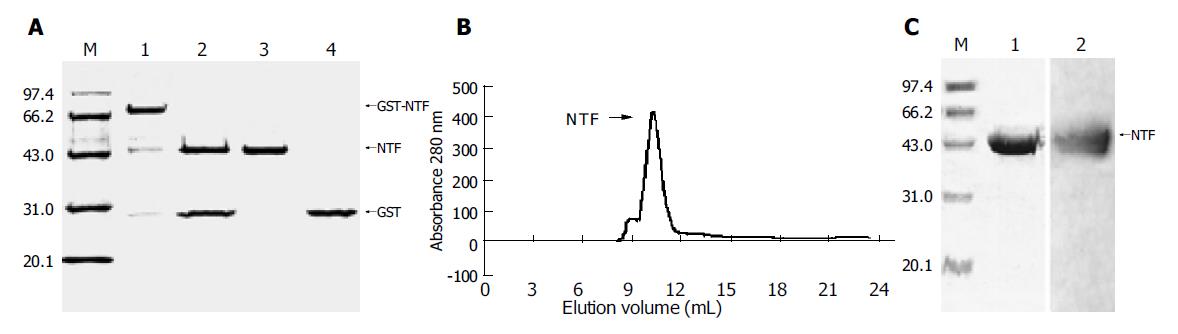
Figure 2 Expression and purification of the N-terminal fragment of gp96 in the GST fusion expression system.
A: The resulting protein N-terminal fragment of gp96 (named after NTF) was separated from the digestion product on a glutathione±Sepharose 4B column to remove GST and GST-3C protease. Lane 1, 10% SDS±PAGE of GST-NTF eluted by reduced glutathione; lane 2, NTF after GST-3C protease digestion (16 h, 5 °C); lane 3, NTF after removing GST and GST-3C protease; lane 4, purified GST as control; M, protein molecular weight markers as indicated in kilo Daltons; B: Gel-filtration analysis of NTF protein. NTF protein subjected to Superdex G75 gel-filtration; C: The proteins from the peak run on 10% SDS-PAGE and Western blot. The standard proteins are superimposed in both A and C.
- Citation: Li HT, Yan JB, Li J, Zhou MH, Zhu XD, Zhang YX, Tien P. Enhancement of humoral immune responses to HBsAg by heat shock protein gp96 and its N-terminal fragment in mice. World J Gastroenterol 2005; 11(19): 2858-2863
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2858