Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.2013
Revised: November 9, 2004
Accepted: December 3, 2004
Published online: April 7, 2005
AIM: To study the correlation between liver fibrosis severity and biliary drainage in patients with choledocholith.
METHODS: A follow-up study on seven patients with liver fibrosis due to choledocholith was made. The data, including biochemical tests (aspartate aminotransferase, alanine aminotransferase) and liver histological features before and after biliary drainage, were collected and studied. The fibrosis severity was scored on a scale from 0 to 3, with 0 denoting none, 1 portal and periportal fibrosis, 2 the presence of numerous fiber septa, and 3 cirrhosis. The average liver fibrosis severity scores of the first and second biopsy were compared with statistical method.
RESULTS: The first, second liver fibrosis severity scores of these seven patients were 2,1; 2,1; 1,0; 1,1; 2,1; 2,1; 1,0 respectively. The results showed that the average liver fibrosis severity score of the second liver biopsy decreased significantly compared with the first liver biopsy (n = 7, t = 4.25, P<0.05).
CONCLUSION: Liver fibrosis due to choledocholith may regress after biliary drainage.
- Citation: Chen ZB, Zheng SS, Hu GZ, Gao Y, Ding CY, Zhang Y, Zhao XH, Ni LM. Liver fibrosis caused by choledocholith to regress after biliary drainage. World J Gastroenterol 2005; 11(13): 2013-2015
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/2013.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.2013
Choledocholith is prevalent in southeast Asia and presents a difficult management problem. The morbidity rate of choledocholith is about 3.2%[1]. However, few cases of choledocholith can lead to liver fibrosis and portal vein hypertension. Seldom reports on the patients with liver fibrosis and portal vein hypertension due to choledocholith are found.
Although, regression of liver fibrosis has been observed after treatment with interferon alpha in patients with chronic hepatitis C, liver fibrosis is usually considered an irreversible process, even when the underlying cause is treated or removed[2]. The exact mechanism is still uncertain. Study in rats showed that liver fibrosis due to chronic obstruction of the biliary duct may regress after the obstruction has been removed[3]. It still remains unknown whether it will happen in human beings too.
Data from a series of eight patients with liver fibrosis due to choledocholith were collected retrospectively from 1988 to 2004. These eight patients were chosen according to the standards followed: (1) with liver fibrosis caused by definite choledocholith reason, excluding virus infection, alcohol or other reasons; (2) with biliary drainage surgery procedure history; (3) with two liver biopsies before and after operation. Three patients accepted second liver biopsy during re-operation for some reasons, such as tumor of stomach, intestine, and abdominal trauma. The other five patients that suffered from repeated right-upper abdominal pain or unknown higher level of aspartate aminotransferase (AST), alanine aminotransferase (ALT), accepted second biopsy for the sake of diagnosis.
The age of these seven patients, including male five, female two, ranged from 25 to 56 years, and the median age was 42.3 years. After the biliary drainage procedure, nearly all patients recovered well except one that suffered from post-operation abdominal cavity infection. No death case was found. The median interval between the first and second histological examination was about 7.6 mo (ranging from 3.5 to 35.6 mo).
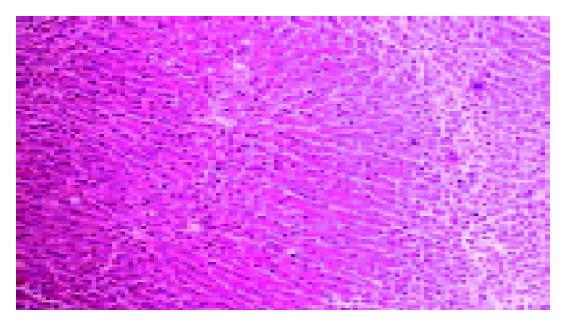
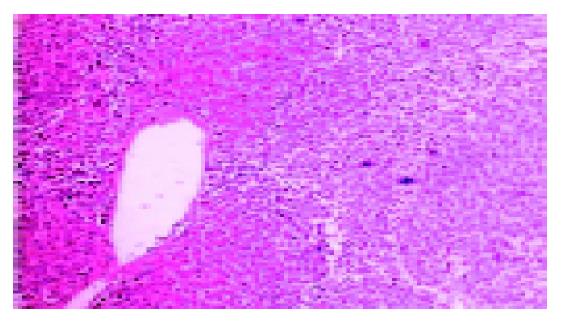
All these eight patients accepted biliary drainage procedure and two liver biopsies. These liver tissues were fixed in 40 g/L formaldehyde and stained with HE. The fibrosis severity was evaluated by pathologists that were unaware of the clinical data. According to its histological feature, the fibrosis severity was scored on a scale from 0 to 3, with 0 denoting none, 1 portal and periportal fibrosis, 2 the presence of numerous fiber septa, and 3 cirrhosis[4].
The data were expressed as mean±SD. These data were analyzed with SPSS.11.0 statistical software. The comparison of two groups dealt with t test. Probabilities less than 0.05 were considered significant.
The data of biochemical tests (including AST, ALT) were collected, the liver fibrosis severity score was evaluated (Tables 1 and 2). The histological features before and after biliary drainage of one and the same patient were presented (Figures 1 and 2). The results showed that the average liver fibrosis severity score of the second liver biopsy decreased significantly compared with that of the first liver biopsy (n = 7, t = 4.25, P<0.05).
| Patient No. | Interval between biopsies (mo) | AST (U/L) | ALT (U/L) | Grade of fibrosis |
| 1 | 6.2 | 302 | 120 | 2 |
| 26 | 25 | 1 | ||
| 2 | 12.5 | 405 | 256 | 2 |
| 12 | 32 | 1 | ||
| 3 | 11.2 | 236 | 32 | 1 |
| 120 | 25 | 0 | ||
| 4 | 48.6 | 125 | 65 | 1 |
| 31 | 23 | 1 | ||
| 5 | 58.3 | 365 | 256 | 2 |
| 65 | 68 | 1 | ||
| 6 | 29.6 | 635 | 486 | 2 |
| 252 | 252 | 1 | ||
| 7 | 44.3 | 295 | 253 | 1 |
| 112 | 98 | 0 |
| Biopsy | Liver fibrosis grade | ||
| 0 | 1 | 2 | |
| The first biopsy | 0 | 3 | 4 |
| The second biopsy | 2 | 5 | 0 |
Although choledocholith is not a rare disease in some Asian countries, it hardly led to liver fibrosis[5]. Most of these patients with liver fibrosis due to choledocholith suffered from repeated right-upper abdominal pain or jaundice. The end term of this disease presents with poor nutrition, ascites, esophageal varices. There are many means, which are proposed to diagnose this disease, but the most effective is liver biopsy[6].
Animal models have provided much information about the structure and function of the liver in chronic biliary obstruction due to choledocholith. Biliary cirrhosis was found in all surviving rats with biliary duct obstruction for 4 wk, and the change of improved hepatic function and the reversal of portal hypertension could be found about 3 wk after decompression operation[7,8]. In another study, the result showed that relief of obstruction after 14 d could lead to normalization of portal pressure and hepatic venous wedge pressure[9].
There are many theories on how biliary duct obstruction caused liver fibrosis. Liver fibrosis is a highly dynamic process in which multiple genes interact with environmental factors. Recent human epidemiologic studies have identified possible polymorphisms in a number of candidate genes that influence the progression of liver fibrosis[10]. The current study has revealed the critical role of P-selectin in the progression of chronic liver fibrosis caused by schistosome parasites[11].
However, few reports on the correlation between liver fibrosis severity and biliary drainage procedure are found. In a study, biliary decompression seemed to alleviate liver cirrhosis and portal hypertension, which were caused by obstruction of the biliary duct[12]. Some causes of chronic obstruction of the biliary duct (ampullary tumors) are rare or are associated with a poor prognosis (pancreatic adenocarcinoma), with death occurring shortly after the biliary drainage procedure. So patients with obstruction of the biliary duct due to choledocholith provide a unique opportunity to study the correlation between liver fibrosis and biliary decompression. Histological examination is very essential for both diagnosis and treatment purpose.
The results of our study showed that secondary biliary fibrosis due to choledocholith may regress after biliary decompression. Since it would be unethical to perform a second liver histological examination without clinical reasons, there were just only seven patients that accepted the second liver biopsy in our study. However, the main defect of our study was selection bias due to few case number and limited clinical data.
Based on the clinical research, we can conclude that the liver fibrosis due to choledocholith may regress after the biliary drainage procedure. Since this disease often presents a very difficult problem for surgeons, many patients have to accept more than a single biliary drainage procedure.However, the mortality rate is high, ranging from 20% to 40%, due to poor conditions of these patients. How and when to perform the operation for this disease always is a challenge for all surgeons. Considering our conclusion, it may suggest that the operation for choledocholith should be performed as soon as possible. Since the liver fibrosis due to choledocholith may regress after biliary decompression, it may also be suggested that we could use a single operation (just remove the stone and build well drainage) instead of so-called radical operation (hepatectomy, splenectomy or devascularization), except which is very essential in some cases, such as accompanied with the bleeding of varice vein or liver lobe infection.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Donato MF, Arosio E, Del Ninno E, Ronchi G, Lampertico P, Morabito A, Balestrieri MR, Colombo M. High rates of hepatocellular carcinoma in cirrhotic patients with high liver cell proliferative activity. Hepatology. 2001;34:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Jiang Y, Liu J, Waalkes M, Kang YJ. Changes in the gene expression associated with carbon tetrachloride-induced liver fibrosis persist after cessation of dosing in mice. Toxicol Sci. 2004;79:404-410. [PubMed] [Cited in This Article: ] |
| 3. | Heistermann HP, Palmes D, Hierlemann H, Ebsen M, Horstmann R, Hohlbach G, Spiegel HU. Reconstruction of bile duct lesions by an autologous vein graft and a bio-degradable endoluminal stent in an animal model: technique and clinical impact. Zentralbl Chir. 2003;128:952-957. [PubMed] [Cited in This Article: ] |
| 4. | Dahab GM, Kheriza MM, El-Beltagi HM, Fouda AM, El-Din OA. Digital quantification of fibrosis in liver biopsy sections: description of a new method by Photoshop software. J Gastroenterol Hepatol. 2004;19:78-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Muriel P, Moreno MG. Effects of silymarin and vitamins E and C on liver damage induced by prolonged biliary obstruction in the rat. Basic Clin Pharmacol Toxicol. 2004;94:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ainsworth AP, Rafaelsen SR, Wamberg PA, Durup J, Pless TK, Mortensen MB. Is there a difference in diagnostic accuracy and clinical impact between endoscopic ultrasonography and magnetic resonance cholangiopancreatography? Endoscopy. 2003;35:1029-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Lorena D, Darby IA, Reinhardt DP, Sapin V, Rosenbaum J, Desmoulière A. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest. 2004;84:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Refik Mas M, Comert B, Oncu K, Vural SA, Akay C, Tasci I, Ozkomur E, Serdar M, Mas N, Alcigir G. The effect of taurine treatment on oxidative stress in experimental liver fibrosis. Hepatol Res. 2004;28:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J, Vendemiale G, Poli G, Viña J, Sastre J. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004;39:711-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003;37:493-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | Wynn TA, Hesse M, Sandler NG, Kaviratne M, Hoffmann KF, Chiaramonte MG, Reiman R, Cheever AW, Sypek JP, Mentink-Kane MM. P-selectin suppresses hepatic inflammation and fibrosis in mice by regulating interferon gamma and the IL-13 decoy receptor. Hepatology. 2004;39:676-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Aithal GP, Haugk B, Das S, Card T, Burt AD, Record CO. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment Pharmacol Ther. 2004;19:391-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |