Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3634
Revised: May 6, 2004
Accepted: May 13, 2004
Published online: December 15, 2004
AIM: H101, an E1B 55 kD gene deleted adenovirus, has been shown to possess oncolysis activity experimentally and proved to be safe in preliminary phase I study. The current study was designed to evaluate its anti-tumor activity and toxicity in combination with chemotherapy in patients with late stage cancers.
METHODS: H101 5.0 × 1011 virus particles were given by intra-tumor injection daily for five consecutive days at every three-week cycle, combined with routine chemotherapy, to one of the tumor lesions of 50 patients with different malignant tumors. Tumor lesions without H101 injection in the same individuals were used as controls. The efficacy and toxicity were recorded.
RESULTS: Forty-six patients were evaluable with a 30.4% response rate. H101 injection in combination with chemotherapy induced three complete response (CR) and 11 partial response (PR), giving an overall response rate of 28.0% (14/50) among intention-to-treat patients. The response rate for the control lesions was 13.0%, including one case with CR and five cases with PR, which was significantly lower than that for the injected lesions (P < 0.05). Main side effects were fever (30.2%) and pain at the injected sites (26.9%). Grade 1 hepatic dysfunction was found in four patients, grade 2 in one patient, and grade 4 in one patient. Hematological toxicity (grade 4) was found in four patients.
CONCLUSION: Intra-tumor injection of the genetically engineered adenovirus H101 exhibits potential anti-tumor activity to refractory malignant tumors in combination with chemotherapy. Low toxicity and good tolerance of patients to H101were observed.
- Citation: Lu W, Zheng S, Li XF, Huang JJ, Zheng X, Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial. World J Gastroenterol 2004; 10(24): 3634-3638
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3634.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3634
The fights against tumors are far from being finished. Biotherapy seems to be a potential anticancer weapon, but still needs strengthening. Engineered virus against cancer is one of the most hopeful therapeutic approaches. There are two different methods: (1) to use replication incompetent viruses as delivery agents for therapeutic genes to access to tumors, and (2) to destroy tumor by using replication-selective oncolytic viruses as therapeutic agents themselves[1,2]. Multiple gene dysfunctions taking part in tumor formation have been known, single gene correction or modification can hardly reverse the malignancy. Viruses engineered for the purpose to replicate only in tumor cells and destroy the cells do not depend on the gene function they take on and have been shown to have great efficacy in both experimental and clinical studies[3-5].
H101 is a recombinant human type-5 adenovirus (Ad5), in which E1B-55 kDs gene has been totally deleted. The H101 virus produced by Shanghai Sunway Biotech, also contains a deletion of 78.3-85.8 μm gene segment in the E3 region. The E1B-55kD gene product is responsible for p53-binding and inactivation[6]. If deleted, the virus would be unable to inactivate p53 for efficient replication in normal cells. However, cancer cells lacking functional p53 would hypothetically be sensitive to viral replication and subsequent cytopathic effects. p53 mutation is the most common genetic abnormality identified in human cancer[7]. This characteristic can be utilized for H101 to identify the target. In vitro and in vivo studies have shown that H101 has anticancer activity, and has been proved to be safe through a five dosage of 5.0 × 107-1.5 × 1012 virus particles (VP)/d within 5 consecutive days in a clinical trial[8]. We carried out this clinical trial to evaluate anti-tumor activity of H101 and its toxicity in combination with chemotherapy in patients with late stage cancers.
Histologically confirmed late stage cancer patients with more than two measurable lesions (at least one could be injected with H101), who had recurrent disease after surgery and/or radiotherapy for the primary tumor, or had progressed at or within 8 wk after completion of chemotherapy and/or radiotherapy, were recruited. Patients had to be ≥ 18 years old, with performance status above grade 2 according to The Eastern Cooperative Oncology Group (ECOG) standard, and life expectancy of ≥ 3 mo. Normal hematological and renal functions were also required. An informed consent was obtained from each patient or from the patient’s legal guardian prior to enrollment. The p53 gene status was not critical for enrollment, because there were factors that inhibited p53 protein function including expression of the human papilloma virus E6 protein or mdm-2 gene amplification[9]. Institutional Review Board approval of the protocol and consent form were granted. This study was also approved by the State Food and Drug Administration of China.
Baseline assessments were made prior to treatment, but these results were not used as enrollment criteria. Baseline blood tests such as complete blood counts, neutralizing antibody titers, electrolytes, blood urea nitrogen, creatinine, and liver function tests were performed. In addition, plain chest radiography, electrocardiogram and type B ultrasonography of upper abdomen were performed.
H101 was formulated as a sterile viral solution in PBS buffer and kept at -20 °C. Each vial contained 0.5 mL of virus solution with 5 × 1011 VP and titered < 1:60 TCID50. Sterile purified lots of virus were produced for human clinical use by Shanghai Sunway Biotech (Shanghai, China), and tested for the titer, sterility, and general safety by National Institute For the Control of Pharmaceutical and Biological Products (Beijing, China).
In each patient, the most symptomatic and/or largest tumor mass was injected with H101, and the patient was treated together with routine systemic chemotherapy simultaneously. The tumor for injection was mapped into five equally spaced sections. Local anesthesia was applied to the skin as needed. The tumor was injected with 5 × 1011 virus particles into one section per day for 5 consecutive days, and these injections were repeated every 3 wk as one treatment cycle. The suspension volume of saline used for H101 administration was normalized to 30% of the estimated volume of the tumor mass to be injected. Tumor volume was estimated as: 1/2 (maximal transverse diameter × maximal vertical diameter × depth).
Tumor masses were measured serially by either physical examination or radiographic scanning (computed tomography or magnetic resonance imaging), whichever the principal investigator deemed most accurate for the measurement of the injected tumor mass. In general, superficial lesions were measured by physical examination, and deep tumors were measured most accurately by radiographic scanning. The tumor mass injected with H101 (injected lesion) and non-injected lesion were evaluated independently. Tumor measurements were performed either every 3 wk (physical examination) while patients were on active study treatment. After treatment completion, patients’ tumor (s) were assessed every 4 wk or sooner if signs/symptoms of progression became evident. Radiographic scanning was assessed by independent radiologists, who were not investigators on the study. The degree of response within injected tumors was categorized as follows: complete regression (CR), complete disappearance of measurable tumor; partial regression (PR), ≥ 50% but < 100% decrease in cross-sectional tumor area; minor response (MR), < 50% but ≥ 25% decrease in tumor area; stable disease (SD), < 25% decrease or 25% increase in tumor area; and progressive disease (PD), ≥ 25% increase in tumor area versus the baseline area. Toxicity was assessed using the National Cancer Institute Toxicity Criteria.
Neutralizing antibody titers were repeated at the end of each cycle, and viral dissemination in blood was tested immediately after injection on d 5 and d 22 for each cycle. The routine blood tests were repeated every week. Fine-needle aspirate biopsies at the injected sites on day 22 of the first treatment cycle were optional, based on patients’ consent because of ethical considerations. These biopsies were analyzed for type Ad5 coat protein by immunohistochemistry.
The blood taken before and one day after injection were collected for PCR detection of H101 genomes (the amplicon overlaps the E1B region deletion and does not detect wild-type adenovirus sequences). The left primer was 5’ctggcgcagaagtattccat3’, at Tm 60.24 °C and the right primer was 5’gtcacatccagcatcacagg3’, at Tm 60.12 °C. Viral DNA was extracted from samples, using the Sangon DNA mini kit (Shanghai, China). The amplification procedure was: at 94 °C for 10 min, then 94 °C for 60 s, 55 °C for 45 s, 72 °C for 60 s for 35 cycles; then at 72 °C, for 10 min. The products were analyzed by 10 g/L agarose electrophoresis. The lower limit of detection was 100 particles of H101 per microlitre plasma.
Triplicate plasma (5 μL) taken before and on d 22 after injection were collected, and tested for Ad-specific antibodies according to the procedures provided by Jingmei Biotech (Shenzhen, China). The absorbance at 450 nm was read on a Bio-Rad Model 550 microplate reader. The positive results were those above or equal to the average of A450nm negative control plus 0.10. Otherwise, the samples were defined as negative.
Injection site fine-needle aspiration biopsies were formalin-fixed, paraffin-embedded and cut into sections. Sections were then deparaffinized and hydrated. Slides were subjected to antigen retrieval at 120 °C for 10 min in citrate buffer and incubated with an Ad5 monoclonal antibody (NeoMarker, America) for 90 min at room temperature. This was followed by incubation with a biotinylated goat anti-mouse secondary antibody, and the streptavidin/horseradish peroxidase conjugate, then mounted in DPX mounting medium (BDH Chemicals, America). The percentage of brown-stained cells (positive for Ad5) was determined by counting the cells under high-power magnification (× 40) of microscope. The average percentage of three high-power field assessments was then calculated. Tumors that had greater than 10% of positively stained cells were considered to be Ad5 positive.
All patients enrolled were calculated under the ITT principle. The rates were compared by χ2 test.
Totally, 50 patients were enrolled, including 18 with head and neck cancer, eight esophageal cancer, five gastric cancer, five lung cancer, three colorectal cancer, three breast cancer, three soft tissue sarcoma, two malignant melanoma, one ovarian cancer, one lymphoma and one chordoma. Most cancers were at end-stage. The head and neck cancer and esophageal cancer enrolled were all squamous carcinoma. Seventy percent of patients were males. The median age was 52 years. All patients had ECOG Performance Status of grade 0-2. Thirty-nine (78%) patients had received pretreatment before, and 31 (62%) had received more than two kinds of treatment. The tumor mass had a median cross-sectional area of 12.5 cm2 (range, 1.43-360 cm2) (Table 1).
| Characteristic | |
| Age (yr) | |
| Median | 52 |
| Range | 18-76 |
| Sex | |
| Male (%) | 35 (70%) |
| Female (%) | 15 (30%) |
| ECOG Performance Status | |
| Grade 0 | 15 (30%) |
| Grade 1 | 21 (42%) |
| Grade 2 | 14 (28%) |
| Pretreatment | |
| Total | 39 (78%) |
| Surgical | 24 (48%) |
| Chemotherapy | 37 (74%) |
| Radiotherapy | 20 (40%) |
| Biotherapy | 8 (16%) |
| Two or more treatment | 31 (62%) |
| Tumor size (cm2) | |
| Median | 12.5 |
| Range | 1.43-360 |
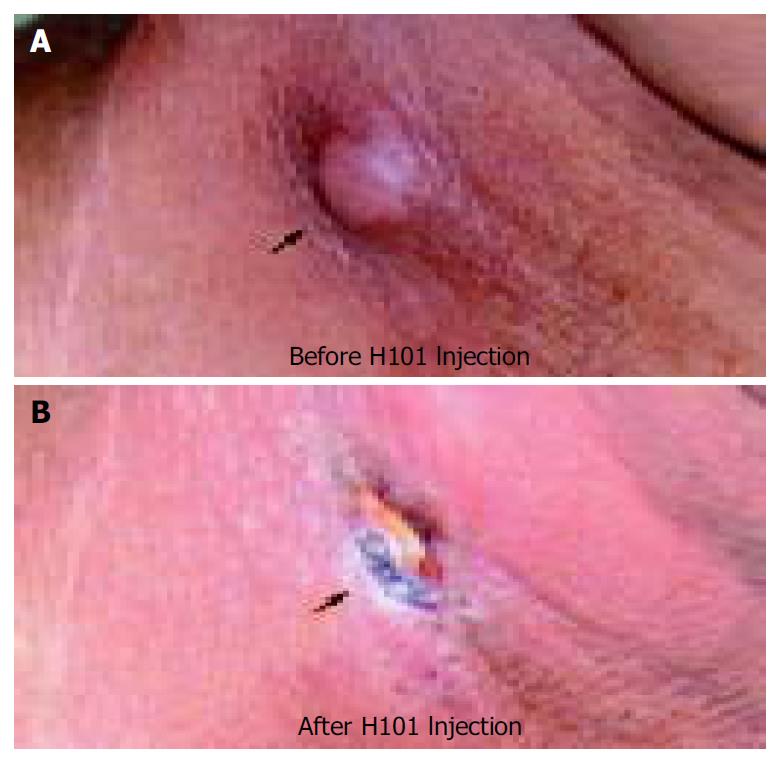
Overall, 46 patients were evaluable. The response rate (CR + PR) among these patients was 30.4% (14/46), and the overall response rate according to ITT principle was 28.0%. For the control lesions, the response rate was 13.0%, which was significantly lower than the H101 treated lesions (χ2 = 4.08, P < 0.05) (Table 2). In the 14 cases with effective H101 injection, there were one CR, three PRs, two MRs, three SDs, and five PDs for the control lesions, respectively. In these patients, combination of H101 injection with chemotherapy was more effective than chemotherapy alone (χ2 = 15.6, P < 0.001). The response rates to H101 injection combined with chemotherapy were different, no effect for gastric cancer was found in this study (Table 3). Figure 1 shows regression of the injected lesion in a patient with head and neck cancer.
| Lesion | n | Median area (cm2) | Efficacy | Response rate (%) | ||||
| CR | PR | MR | SD | PD | ||||
| H101 injection | 46 | 12.5 | 3 | 11 | 11 | 13 | 8 | 30.4 |
| Control | 46 | 11.3 | 1 | 5 | 7 | 21 | 12 | 13.0 |
The most frequent adverse reaction was fever (30.2%), injection site pain (26.9%), flu-like symptoms (26.4%), nausea and vomiting (34.0%), leucopenia (49.1%), liver dysfunction (5.7%), alopecia (13.2%) (Table 4). Fever was moderate, which appeared at about 12 h post H101 injection, persisted for 2-4 h, and then returned to normal without treatment. There was a significant difference in the regression rate between patients with fever (69.2%, 9/13) and those without fever (21.2%, 7/33) (χ2 = 9.48, P < 0.005).
| Adverse event | Grade | Total (%) | |||
| I | II | III | IV | ||
| Fever | 10 | 5 | 1 | 0 | 16 (30.2) |
| Injection site pain | 12 | 2 | 0 | 0 | 14 (26.4) |
| Nausea and vomiting | 13 | 5 | 0 | 0 | 18 (34.0) |
| Leucopenia | 12 | 7 | 3 | 4 | 26 (49.1) |
| Liver dysfunction | 2 | 0 | 0 | 1 | 3 (5.7) |
| Flu-like symptom | 13 | 2 | 0 | 0 | 15 (28.3) |
| Alopecia | 3 | 3 | 1 | 0 | 7 (13.2) |
Fourteen patients were tested for the Ad-specific neutralizing antibody. Three (21.4%) of them were positive at baseline. Another six turned to be positive on day 22. Two patients positive at the baseline and two negative patients experienced tumor regression, and thus there was no correlation between baseline neutralizing antibody titers and induction of tumor response. Sixteen patients were tested for plasma H101 viral genome before injection and 30 min after. Only six cases were positive after injection (Table 5). All these patients were positive for blood Ad-specific neutralizing antibody on d 22.
| Before injection | After injection | |||
| Negative | Positive | Negative | Positive | |
| Ad neutralizing titer | 11 | 3 | 5 | 9 |
| Plasma H101 PCR | 11 | 0 | 7 | 4 |
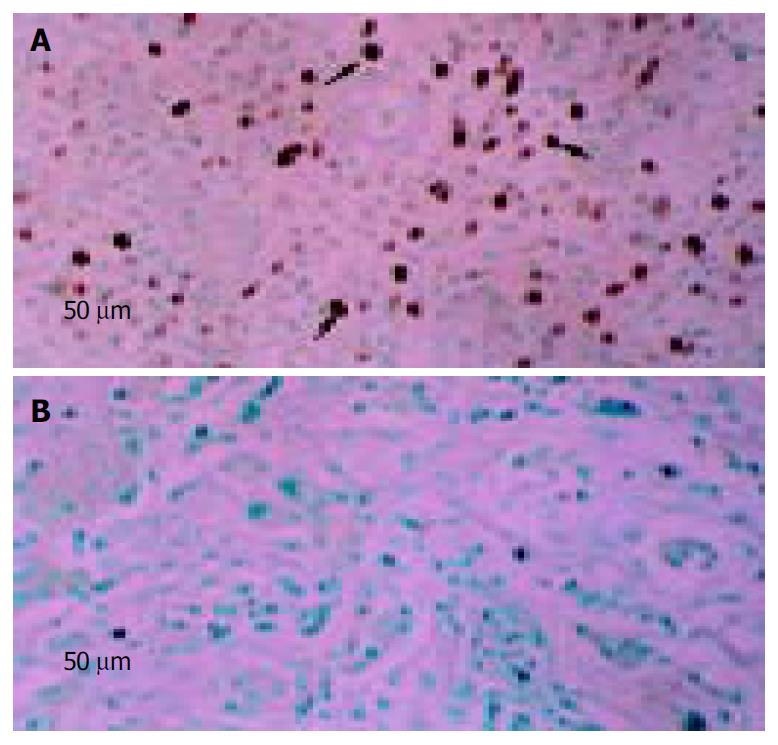
Totally, three fine-needle aspiration biopsies of tumor were obtained at the end of treatment on d 22 or d 44, and detected for Ad5 coat protein by immunohistochemistry for adenovirus presence. Two of them were positive (Figure 2).
Selective replication of E1B deleted adenovirus in the p53 dysfunctional human cancer for cancer therapy is one of promising treatment approaches. Its safety has been shown in a number of clinical trials[5,10-12]. Although anticancer activity of the virus has been proved, the clinical efficacy is still not predominant. Therefore, the oncolytic ability needs to be enhanced. Current studies are focusing on arming these viruses with therapeutic genes to increase it potency.[13-15]
But before that, the virus itself can be reinforced by augmentation or elimination of specific viral functions to enhance the anticancer efficacy. To enhance the virus-induced host anti-tumor immune response is one of the key points. However, the roles of the immune response to virotherapy are profound. Cutting down the functions of the virus to escape from immune surveillance can impede the spread of viral infection on the one hand, but augment tumor cell destruction through the recruitment of T cells “vaccinated” against tumor antigens on the other[16]. The E3 region is related to the inhibition of host immunity, which enhances the virus replication and spread in tumor[17]. But this is not necessary for intra-tumor injection of oncolytic viral. The virus replication and spread effect can be enhanced by repeated injection. By sacrificing the spread ability, the virus may activate the host immune response to virus infected tumor cells and help the host immune system to recognize tumor cells themselves, and thus may benefit patients under such therapy. Metastasis is prevalent in malignant tumor patients, which is the main cause of treatment failure or even death. Moreover, patients may have more than one tumor lesion, and the lesion that cannot be injected could exist. Therefore, the ability of activating the host immune response seems crucial. So treatment with the E3 region deleted adenovirus, H101, may have additional benefit to patients.
The main purpose of this pilot study was to test the effect of H101 on a wide type of advanced cancers. Results showed that the total response rate was only 28.0% under the ITT principle, which was significantly higher than the lesions that received chemotherapy alone (P < 0.05). This indicates that H101 may have potential anticancer activity. The total regression rate observed is not salient for the treatment. This may be due to the late stage of the diseases, and most of the patients had been vigorously treated previously but failed at last. The other reason is the wide enrollment of the tumor types, some of which might not be sensitive to H101. For instance, gastric cancer showed no response.
However, some patients presented notable therapeutic efficacy without grievous adverse reactions. Moreover, in those who had fever during H101 injection, the efficacy was significantly higher than those who did not have fever (P < 0.005). Although there is no enough evidence to estimate the effect of H101 on host immunity to tumors, our results suggest that there is a relationship between the immune reaction to H101 and the efficacy, which was not well recognized in previous studies. In the beginning of last century, it was noticed that patients with various malignancies experienced spontaneous tumor regression after rabies vaccination, a viral illness or even bacterial infection[18,19]. In these cases, virus infection may activate the host immune system, and elevated cell-mediated immunity may play a role in the tumor regression. But the mechanism is still unclear. On the basis of those results, immune modulation strategies should be further studied and developed.
Our study also shows that H101 intra-tumor injection is well tolerated. No severe toxicity was observed, and the main adverse reactions that related to H101 were injection site pain, nausea, fever and flu-like symptoms. Fever and flu-like symptoms were obviously caused by the virus injection and consequently transitory viraemia. H101 presence did not cause severe inflammation in peritumoral normal tissue, despite multiple directive injection. Thus, H101 may benefit the patient without adding severe affliction in clinical application.
Treatment for cancers with the recombinant oncolytic adenovirus is hopeful, but still immature. Experiences should be accumulated before it is applied in cancer therapy. Since patients enrolled in our clinical trial were in their end-stage of diseases, there were difficulties in patient selection and unifying the chemotherapy drugs due to ethical consideration, and immunosuppression was prevalent in those patients. The clinical benefit of intra-tumor injection with H101 should be further determined in randomized trials and, possibly, in earlier stage patients. The dosage, medication methods, treatment cycle and combined chemotherapy or immunotherapy should be explored in further studies as well. Genetically engineered and reinforced viruses may become a novel therapeutic platform for the treatment of cancers.
Edited by Xia HHX and Zhu LH Proofread by Xu FM
| 1. | Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, Haynes H, Wadler S. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9:693-702. [PubMed] [Cited in This Article: ] |
| 4. | Habib NA, Mitry RR, Sarraf CE, Jiao LR, Havlík R, Nicholls J, Jensen SL. Assessment of growth inhibition and morphological changes in in vitro and in vivo hepatocellular carcinoma models post treatment with dl1520 adenovirus. Cancer Gene Ther. 2002;9:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hamid O, Varterasian ML, Wadler S, Hecht JR, Benson A, Galanis E, Uprichard M, Omer C, Bycott P, Hackman RC. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:1498-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470-1473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 250] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5532] [Cited by in F6Publishing: 5428] [Article Influence: 164.5] [Reference Citation Analysis (0)] |
| 8. | Yuan ZY, Zhang L, Li S, Qian XZ, Guan ZZ. [Safety of an E1B deleted adenovirus administered intratumorally to patients with cancer]. Aizheng. 2003;22:310-313. [PubMed] [Cited in This Article: ] |
| 9. | Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW, Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53:2231-2234. [PubMed] [Cited in This Article: ] |
| 10. | Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289-298. [PubMed] [Cited in This Article: ] |
| 12. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 820] [Cited by in F6Publishing: 753] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 13. | Hermiston TW, Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002;9:1022-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Stubdal H, Perin N, Lemmon M, Holman P, Bauzon M, Potter PM, Danks MK, Fattaey A, Dubensky T, Johnson L. A prodrug strategy using ONYX-015-based replicating adenoviruses to deliver rabbit carboxylesterase to tumor cells for conversion of CPT-11 to SN-38. Cancer Res. 2003;63:6900-6908. [PubMed] [Cited in This Article: ] |
| 15. | Bauzon M, Castro D, Karr M, Hawkins LK, Hermiston TW. Multigene expression from a replicating adenovirus using native viral promoters. Mol Ther. 2003;7:526-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7:106-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Benedict CA, Norris PS, Prigozy TI, Bodmer JL, Mahr JA, Garnett CT, Martinon F, Tschopp J, Gooding LR, Ware CF. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J Biol Chem. 2001;276:3270-3278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Chabalgoity JA, Dougan G, Mastroeni P, Aspinall RJ. Live bacteria as the basis for immunotherapies against cancer. Expert Rev Vaccines. 2002;1:495-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lamon EW, Hale P, Whitten HD. Antibody-dependent, cell-mediated cytotoxicity with autochthonous lymphocytes and sera after infection with Moloney sarcoma virus. J Natl Cancer Inst. 1976;56:349-355. [PubMed] [Cited in This Article: ] |