Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2697
Revised: September 23, 2003
Accepted: October 29, 2003
Published online: September 15, 2004
AIM: To explore the effects of endothelin-1 (ET-1) on hepatic stellate cells (HSCs) DNA uptake, DNA synthesis, collagen synthesis and secretion, inward whole-cell calcium concentration ([Ca2+]i) as well as the blocking effect of verapamil on ET-1-stimulated release of inward calcium (Ca2+) of HSC in vitro.
METHODS: Rat hepatic stellate cells (HSCs) were isolated and cultivated. 3H-TdR and 3H-proline incorporation used for testing DNA uptake and synthesis, collagen synthesis and secretion of HSCs cultured in vitro; Fluorescent calcium indicator Fura-2/AM was used to measure [Ca2+]i inward HSCs.
RESULTS: ET-1 at the concentration of 5 × 10-8 mol/L, caused significant increase both in HSC DNA synthesis (2247 ± 344 cpm, P < 0.05) and DNA uptake (P < 0.05) when compared with the control group. ET-1 could also increase collagen synthesis (P < 0.05 vs control group) and collagen secretion (P < 0.05 vs control group). Besides, inward HSC [Ca2+] i reached a peak concentration (422 ± 98 mol/L, P < 0.001) at 2 min and then went down slowly to165 ± 51 mol/L (P < 0.01) at 25 min from resting state (39 ± 4 mol/L) after treated with ET-1. Verapamil (5 mol/L) blocked ET-1-activated [Ca2+]i inward HSCs compared with control group (P < 0.05). Fura-2/AM loaded HSC was suspended in no Ca2+ buffer containing 1 mol/L EGTA, 5 min later, 10-8 mol/L of ET-1 was added, [Ca2+]i inward HSCs rose from resting state to peak 399 ± 123 mol/L, then began to come down by the time of 20 min. It could also raise [Ca2+]i inward HSCs even without Ca2+ in extracellular fluid, and had a remarkable dose-effect relationship (P < 0.05). Meanwhile, verapamil could restrain the action of ET-1 (P < 0.05).
CONCLUSION: Actions of ET-1 on collagen metabolism of HSCs may depend on the transportation of inward whole-cell calcium.
- Citation: Guo CY, Wu JY, Wu YB, Zhong MZ, Lu HM. Effects of endothelin-1 on hepatic stellate cell proliferation, collagen synthesis and secretion, intracellular free calcium concentration. World J Gastroenterol 2004; 10(18): 2697-2700
- URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2697
Hepatic fibrosis associated with the activation of hepatic stellate cells (HSCs), the major source of extracellular matrix (ECM) proteins[1]. It is generally believed that HSCs are the main cells producing ECM, from resting state to active myofibroblasts, which is the key point of formation and development of hepatic fibrosis[2-6]. Endothelin-1(ET-1) is currently known as a polypeptide with a stronger activity to contract blood vessel. So, based on prophase researches[7-9], we chose ET-1 to observed its direct effect on DNA ingestion and synthesis as well as collagen synthesis and secretion of HSCs in cultivating. Meanwhile, as we know that Ca2+ is an important intracellular messenger, relate to HSC proliferation and ECM synthesis[10-13]. The effects of ET-1 on regulation and intracellular [Ca2+]i of HSCs isolated and cultivated in vitro were studied.
Animals Wistar male rats, weighting (450 ± 50) g, were provided by Shanghai Experimental Animals Center of Chinese Academy of Sciences.
Reagents ET-1, calcium fluorescence probes Fura-2/AM, Triton X-100, pronase, trypsin, DMEM, DAB-H2O2 were from Sigma; verapamil from Knoll; collagenase from Medical Industry Academy of Shanghai; RPMI 1640 from Gibco; HEPES from EMK; 3H-L-proline from Academy of Atomic Energy in China (66.6 GBq/mmoL, radioactivity purity > 90%). 3H-TdR was from Institute of Atomic Energy in Shanghai (814 GBq/mmoL, radioactivity purity > 95%).
Isolation and cultivation of rat HSCs Rat HSCs were isolated referring to Knook[14-17]. Rats were anaesthetized with pentobarbitone (200 mg/kg) by abdominal injection, then heparin sodium (10 mg/kg) was injected into the caudal vein. The abdominal cavity was opened and portal vein and dorsal vein were exposed. Blood was released through vein and D-Hank’s solution was perfused (20-25 mL/min) until pale yellow appeared. Liver was taken out and undergone extracorporeal circulation when perfusion fluid was changed to GBSS containing 0.5 g/L pronase E, 0.5 g/L collagenase and 10 mmoL HEPES. Circle perfusion was performed for 30 min (15 mL/min). Liver was taken out and cut to pieces, then put into GBSS containing 0.25 g/L pronase E, 0.25 g/L collagenase and 10 mmol/L HEPES, shocked at 37 °C for 30 min, little suspended deposit was put in culture media on the top of three-layer density gradient centrifugation fluid containing 80 g/L and 130 g/L metrizamide, 2800 r/min centrifugation for 20 min, Cells were sucked between top layer and 80 mL/L density layer. DMEM containing 200 mL/L calf serum was used to regulate the number of cells to 1 × 105/mL.
DNA and collagen synthesis of HSCs HSCs in 2 to 4 th generation were digested by pancreatin and cultured with DMEM supplemented with 100 g/L calf serum and 100 mL/L horse serum. Cells were adjusted to 1 × 10-8/mL and inoculated on a 48-well plate, cultured for 24 h, then different concentration of ET-1 and the same dosage of drug was added, respectively and triplicated for each concentration. 3H-TdR and 3H-proline were used to assay the incorporation.
HSC ingestion of DNA3H-TdR 18.5 GBq/mmoL was added at 10, 20, 30 and 60 min respectively, washed 3 times with PBS of 1 × 105 mmol/L, centrifuged 1000 r/min 10 min, the top layer fluid was removed, 2 mL of 100 g/L TCA was added and centrifuged 1000 r/min 10 min again. Top layer fluid was collected and deposited, washed 3 times with 800 mL/L ethanol at 4 °C. The top layer fluid was removed and dried in vacuum. 1 mol/L NaOH was added to lyse the deposit and 1N HCl was used to adjust pH to 7.0 Radioactivity of specimens was measured on Beckman scintillation counter.
Collagen secretion of HSC In experiment of 3H-TdR, before transferred to F49 filter paper, 1 mL culture media was taken out and put into a tube. A 5 mmol/L acetic acid was used to adjust pH to 2 to 3, then 25 μL of 2.5 g/L pepsin was added to digest. A 50 μL of proline was added at 4 °C for 3 h, l, 1.2 mol/L trichloroacetic acid was fixed for 2 h, transferred to F4a filter paper, closed with saline, 0.6 mol/L trichloroacetic acid was used again, then bleached with anhydrous alcohol, baked at 80 °C. Radioactivity of specimens was measured on YSJ-75 liquid scintillation counter.
[Ca2+]i in Fura-2/AM loaded HSC HSCs were cultured on a rectangle glass when HSCs grew and covered the glass. Then cells were taken out of the glass and RPMI 1640 containing Fura-2/AM (10 nmol/L) was added to incubate at 37 °C for 50 min, D-Hank’s solution was used to wash extracellular free Fura-2/AM and incubated for another 30 min, 1 g/L trypsin was used to digest the cells and the number of cells was adjusted to 106/mL by buffer.
Fluorescence spectrum About 2 mL of Fura-2/AM loaded HSCs was suspended for the test with a fluorescence spectrophotometer. Raster (EX) 5 nm, radiate raster (EM) 10 nm were excited at a middle scan speed (32 mm/min), excitation light scan ranged 300-400 nm, emission light scan ranged 440-540 nm.
Intracellular fluorescence intensity Fluorescence intensity F was detected first (laser wave-length 340 nm, EX 5 nm, emission wave-length 510 nm, EM 10 nm), then different concentrations of ET-1 and verapamil and EGTA (last concentration 8 mmol/L) were added for the detection of minimum fluorescence intensity (F min).
Calculation of [Ca2+]i Intracellular [Ca2+]i (nmol/L) = kd (F - F min)/(F max - F). Kd is a dissociation constant to Fura-2/Ca2+ compound which equals to 224 nmol/L.
Statistical analysis Variance homogeneity tests were used to make comparisons.
Trypan blue staining revealed an activity above 90% for HSCs. The purity of HSCs was more than 80% assessed by fluorescence microscope. The nuclei of HSC were stained blue among the desmin-positive satellite cells.
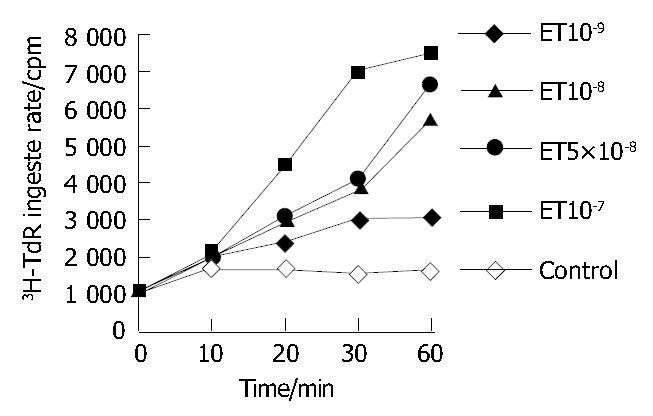
As shown in Table 1, ET-1 could accelerate 3H-TdR incorporation into HSCs and HSC DNA synthesis and proliferation (P < 0.05), in a concentration-dependent manner.
ET-1 could accelerate the rate of HSC ingested DNA, the rate increased with the time prolonged (P < 0.05 or P < 0.01, Figure 1).
ET-1 could accelerate 3H-Proline incorporation into HSCs and collagen synthesis at the concentration of 5 × 10-8 mol/L (P < 0.05), in a concentration-dependent manner.
As shown in Table 1, ET-1 could remarkably accelerate HSC collagen secretion compared with the control group (P < 0.05).
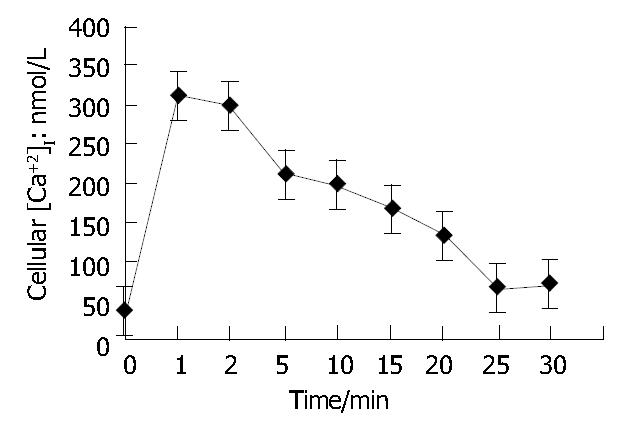
As shown in Figure 2, when ET-1 was added to the suspension of Fura-2/AM loaded HSCs and kept for 25 min (n = 3), [Ca2+]i in HSCs rose from (39 ± 4) mol/L (resting state) to (165 ± 51) mol/L (P < 0.01) and rose to peak (422 ± 98) mol/L ( P < 0.001) after another 2 min, then it began to go down slowly and remained a higher concentration even after another 18 min compared with the resting [Ca2+]i (P < 0.01). It suggested that the effect of ET-1 on [Ca2+]i in HSCs could be divided into 2 phases, a fast phase (I P) and a slow phase (II P).
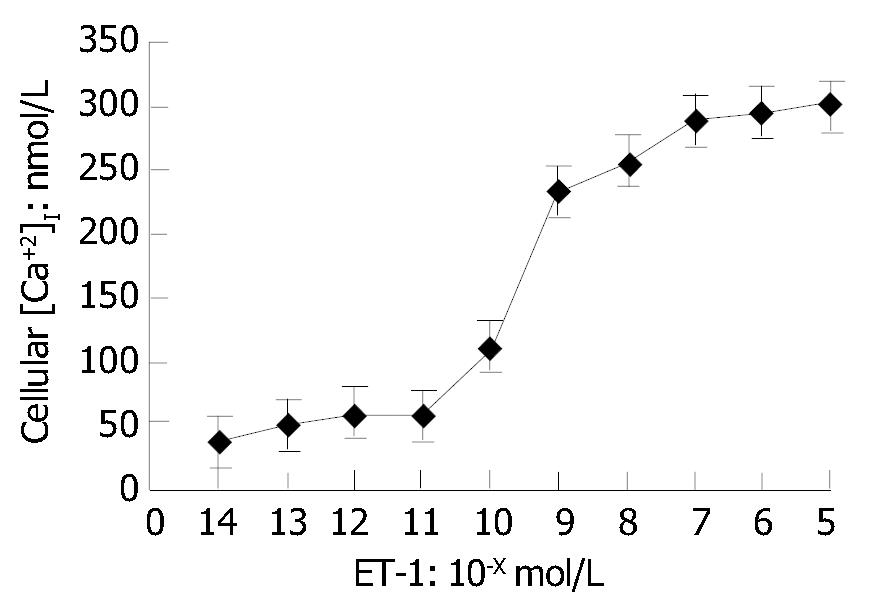
As shown in Figure 3, [Ca2+]i in HSCs was in a ET-1 concentration-dependent manner. No change of [Ca2+]i occurred in HSCs when ET-1 was less than 10-11 mol/L. [Ca2+]i reached its peak in a ET-1-dose-dependent manner when ET-1 was greater than 10-9 mol/L.
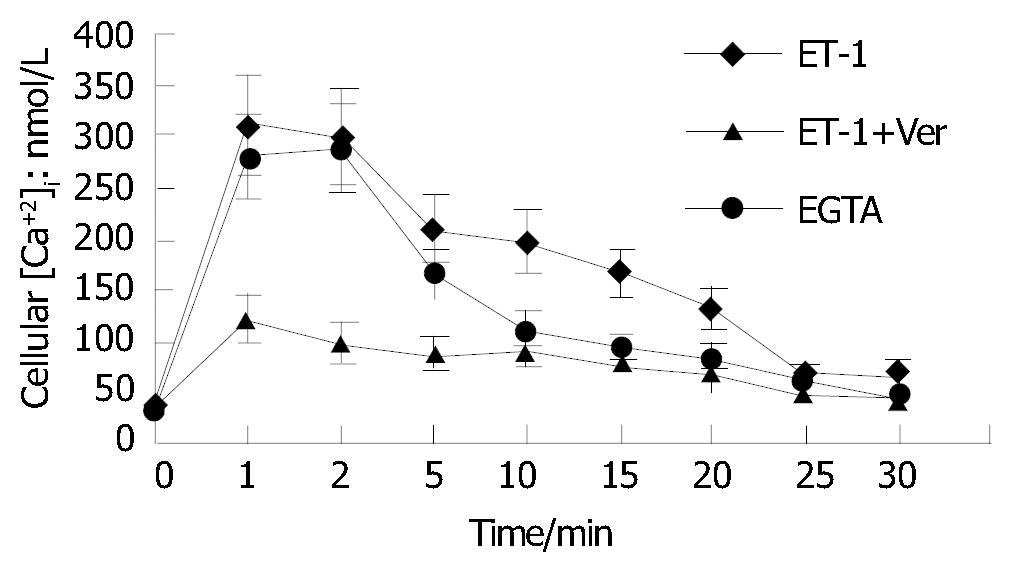
As shown in Figure 4, calcium channel blocking agent verapamil (5 μmol/L) could significantly restrain I P and II P effects on [Ca2+]i in HSCs excited by ET-1 compared with control group (P < 0.05). Fura-2/AM loaded HSCs suspended in Ca2+-free buffer containing 1 mol/L EGTA made [Ca2+]i in HSCs raise from resting state to peak (399 ± 123) mol/L, then go down to (49 ± 17) mol/L at the time of 20 min when first treated with 10-8 mol/L of ET-1, suggesting that Ca2+-free buffer had no remarkable effect on I P of [Ca2+]i in HSCs excited by ET-1 but completely blocked II P.
HSCs were first detected by Ito and Nemoto in 1952, which provided a new way to study episode mechanism of hepatic fibrosis and deepened the cognition of hepatic fibrosis from an angle of source cells of collagen production[2-6,15,16]. HSCs is also named Ito cell, VitA storing cell, liver antrum around cell, fat-storing cell, and is one of the liver interstitial cells. The main function of HSC is to store and metabolize VitA. It has been found to be able to synthesize and secrete ECM and synthesize collagenase[2-6]. When hepatic fibrosis occurred, HSC turned into fibroblasts or myofibroblasts that were the cause of liver synthesis of ECM. This change of HSC was called activation or conversion[2,3]. It has been certificated that interstitial cells especially HSCs are the main cells which producte collagen when hepatic fibrosis occurs. So it has become a central link in hepatic fibrosis occurrence mechanism.
ET distributes widely in liver and portal vein system, and has important biological effects on liver[18-24]. This experiment showed that ET could remarkably accelerate HSC proliferation, DNA synthesis, collagen synthesis and secretion. It is thus clear that ET-1 had double roles during hepatic fibrosis, accelerating not only HSC synthesis of collagen but also selective excretion of collagen. Besides[25,26], endothelial cells in hepatic sinusoid secrete endothelins that can activate HSCs. It has been reported that ET could raise [Ca2+]i in smooth muscle cells[27-29]. This study showed that ET-1 could raise [Ca2+]i in HSCs and appeared double phase reaction, fast phase and slow phase. Both phases had a dose-dependent manner. It turns out that when the cells are at resting state, if there is extracellular Ca2+, the [Ca2+]i in HSCs will be higher than that without extracellular Ca2+. ET-1 can remarkably raise [Ca2+]i in HSC with or without extracellular Ca2+. It implies that ET-1 can accelerate HSC release of intracellular Ca2+.
Three different ways have been found to elevate [Ca2+]i[30-33]. Plenty of calcium flows into cell through Ca2+ channel, Ca2+-ATP enzyme or Na+-Ca2+ changing system is restrained which can transfer Ca2+ out of cells; Ca2+storing systems such as mitochondrion and endoplasm increase Ca2+. We used Ca2+-free buffer and found it had no effect on [Ca2+]i in I P in HSC excited by ET-1 but could block [Ca2+]i in II P. It implies that elevated [Ca2+]i in I P is caused by increased Ca2+ stored in cells, while elevated [Ca2+]i in II P is caused by Ca2+ flowing out of cells. It has been currently accepted by some of scholars that the raise of Ca2+ in HSC is through the way of phospholipase C (PLC)-inositol triphosphate (IP3)-diacylgcerol (DAG)[34-51]. ET-1 excites PLC on cell membrane through G protein that makes 4,5-biphosphate inositol divide into IP3 and DAG-IP3. Mitochondrion, endoplasm and sarcoplasm that make Ca2+ in cell release to cytoplasm and increase free [Ca2+]i in cells. IP3 works only a very short time, and is quickly converted to IP4 by special enzymes. So peak I P lasts for a very short time, but IP4 can accelerate the opening of Ca2+ channel on cell membrane, which makes an increase of calcium flowing out of cells and at last results in a fast raise of [Ca2+]i in cells.
Physiological and pathological significance of elevated free Ca2+ in HSCs excited by ET is still not clear. Maybe it could participate the series of signals in cells and physiological effect of ET[25,27]. In conclusion, ET-1 can remarkably accelerate HSC proliferation, collagen synthesis and secretion, increase of [Ca2+]i in HSC and of release of Ca2+ in cells, thus accelerating proliferation of fibrous tissues and repair of injury tissues.
Edited by Wang XL Proofread by Xu FM
| 1. | Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206-209. [PubMed] [Cited in This Article: ] |
| 2. | Kawada N. The hepatic perisinusoidal stellate cell. Histol Histopathol. 1997;12:1069-1080. [PubMed] [Cited in This Article: ] |
| 3. | Schuppan D, Popov Y. Hepatic fibrosis: from bench to bedside. J Gastroenterol Hepatol. 2002;17 Suppl 3:S300-S305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Marra F, Pinzani M. Role of hepatic stellate cells in the pathogenesis of portal hypertension. Nefrologia. 2002;22 Suppl 5:34-40. [PubMed] [Cited in This Article: ] |
| 5. | Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Ramadori G, Saile B. Mesenchymal cells in the liver--one cell type or two? Liver. 2002;22:283-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Hasegawa T, Kimura T, Sasaki T, Okada A. Plasma endothelin-1 level as a marker reflecting the severity of portal hypertension in biliary atresia. J Pediatr Surg. 2001;36:1609-1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Tsuchiya Y, Suzuki S, Inaba K, Sakaguchi T, Baba S, Miwa M, Konno H, Nakamura S. Impact of endothelin-1 on microcirculatory disturbance after partial hepatectomy under ischemia/reperfusion in thioacetamide-induced cirrhotic rats. J Surg Res. 2003;111:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Hasselblatt M, Bunte M, Dringen R, Tabernero A, Medina JM, Giaume C, Sirén AL, Ehrenreich H. Effect of endothelin-1 on astrocytic protein content. Glia. 2003;42:390-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Raue F. Epidemiology of medullary thyroid carcinoma. Recent Results Cancer Res. 1992;125:47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Raue F, Zink A. Measurement of free cytosolic calcium in single cells: method and application. Methods Find Exp Clin Pharmacol. 1992;14:327-332. [PubMed] [Cited in This Article: ] |
| 12. | Lyall V, Alam RI, Phan TH, Phan DQ, Heck GL, DeSimone JA. Excitation and adaptation in the detection of hydrogen ions by taste receptor cells: a role for cAMP and Ca(2+). J Neurophysiol. 2002;87:399-408. [PubMed] [Cited in This Article: ] |
| 13. | Satin LS, Kinard TA. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine. 1998;8:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Brouwer A, Barelds RJ, de Leeuw AM, Blauw E, Plas A, Yap SH, van den Broek AM, Knook DL. Isolation and culture of Kupffer cells from human liver. Ultrastructure, endocytosis and prostaglandin synthesis. J Hepatol. 1988;6:36-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Hendriks HF, Brouwer A, Knook DL. The role of hepatic fat-storing (stellate) cells in retinoid metabolism. Hepatology. 1987;7:1368-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | de Leeuw AM, McCarthy SP, Geerts A, Knook DL. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984;4:392-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 272] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Knook DL, Seffelaar AM, de Leeuw AM. Fat-storing cells of the rat liver. Their isolation and purification. Exp Cell Res. 1982;139:468-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 191] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Housset C, Rockey DC, Bissell DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci USA. 1993;90:9266-9270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 220] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Yokomori H, Oda M, Ogi M, Kamegaya Y, Tsukada N, Nakamura M, Ishii H. Enhanced expression of endothelin receptor subtypes in cirrhotic rat liver. Liver. 2001;21:114-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Moore K, Wendon J, Frazer M, Karani J, Williams R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992;327:1774-1778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 254] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Serradeil-Le Gal C, Jouneaux C, Sanchez-Bueno A, Raufaste D, Roche B, Préaux AM, Maffrand JP, Cobbold PH, Hanoune J, Lotersztajn S. Endothelin action in rat liver. Receptors, free Ca2+ oscillations, and activation of glycogenolysis. J Clin Invest. 1991;87:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Møller S, Emmeluth C, Henriksen JH. Elevated circulating plasma endothelin-1 concentrations in cirrhosis. J Hepatol. 1993;19:285-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Saló J, Francitorra A, Follo A, Navasa M, Ginès A, Jiménez W, Ginès P, Arroyo V, Rivera F, Rodés J. Increased plasma endothelin in cirrhosis. Relationship with systemic endotoxemia and response to changes in effective blood volume. J Hepatol. 1995;22:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Yokomori H, Oda M, Yasogawa Y, Nishi Y, Ishii H. Signal detection of endothelin receptor subtypes in human cirrhotic liver by a new in situ hybridization method. Med Electron Microsc. 2000;33:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Yokomori H, Oda M, Ogi M, Yoshimura K, Nomura M, Fujimaki K, Kamegaya Y, Tsukada N, Ishii H. Endothelin-1 suppresses plasma membrane Ca++-ATPase, concomitant with contraction of hepatic sinusoidal endothelial fenestrae. Am J Pathol. 2003;162:557-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Oda M, Han JY, Yokomori H. Local regulators of hepatic sinusoidal microcirculation: recent advances. Clin Hemorheol Microcirc. 2000;23:85-94. [PubMed] [Cited in This Article: ] |
| 27. | Gasull X, Bataller R, Ginès P, Sancho-Bru P, Nicolás JM, Görbig MN, Ferrer E, Badía E, Gual A, Arroyo V. Human myofibroblastic hepatic stellate cells express Ca(2+)-activated K(+) channels that modulate the effects of endothelin-1 and nitric oxide. J Hepatol. 2001;35:739-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Reinehr RM, Kubitz R, Peters-Regehr T, Bode JG, Häussinger D. Activation of rat hepatic stellate cells in culture is associated with increased sensitivity to endothelin 1. Hepatology. 1998;28:1566-1577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Shao R, Rockey DC. Effects of endothelins on hepatic stellate cell synthesis of endothelin-1 during hepatic wound healing. J Cell Physiol. 2002;191:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Kriebel ME, Keller B. The unitary evoked potential at the frog nerve-muscle junction results from synchronous gating of fusion pores at docked vesicles. Cell Biol Int. 1999;23:527-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Smogorzewski MJ. Central nervous dysfunction in uremia. Am J Kidney Dis. 2001;38:S122-S128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine. 2000;13:251-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Murthy KS, Zhou H. Selective phosphorylation of the IP3R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G221-G230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86:2863-2867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1654] [Cited by in F6Publishing: 1613] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 35. | Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 645] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 36. | Clozel M, Gray GA, Breu V, Löffler BM, Osterwalder R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun. 1992;186:867-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 303] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992;13:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 413] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 38. | Higuchi H, Satoh T. Endothelin-1 induces vasoconstriction and nitric oxide release via endothelin ET(B) receptors in isolated perfused rat liver. Eur J Pharmacol. 1997;328:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Jouneaux C, Mallat A, Serradeil-Le Gal C, Goldsmith P, Hanoune J, Lotersztajn S. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and Gq in rat liver. J Biol Chem. 1994;269:1845-1851. [PubMed] [Cited in This Article: ] |
| 40. | Kuddus RH, Nalesnik MA, Subbotin VM, Rao AS, Gandhi CR. Enhanced synthesis and reduced metabolism of endothelin-1 (ET-1) by hepatocytes--an important mechanism of increased endogenous levels of ET-1 in liver cirrhosis. J Hepatol. 2000;33:725-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Leivas A, Jiménez W, Bruix J, Boix L, Bosch J, Arroyo V, Rivera F, Rodés J. Gene expression of endothelin-1 and ET(A) and ET(B) receptors in human cirrhosis: relationship with hepatic hemodynamics. J Vasc Res. 1998;35:186-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Gerbes AL, Møller S, Gülberg V, Henriksen JH. Endothelin-1 and -3 plasma concentrations in patients with cirrhosis: role of splanchnic and renal passage and liver function. Hepatology. 1995;21:735-739. [PubMed] [Cited in This Article: ] |
| 43. | Uchihara M, Izumi N, Sato C, Marumo F. Clinical significance of elevated plasma endothelin concentration in patients with cirrhosis. Hepatology. 1992;16:95-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Asbert M, Ginès A, Ginès P, Jiménez W, Clària J, Saló J, Arroyo V, Rivera F, Rodés J. Circulating levels of endothelin in cirrhosis. Gastroenterology. 1993;104:1485-1491. [PubMed] [Cited in This Article: ] |
| 45. | Matsumoto H, Uemasu J, Kitano M, Kawasaki H. Clinical significance of plasma endothelin-1 in patients with chronic liver disease. Dig Dis Sci. 1994;39:2665-2670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Møller S, Gülberg V, Henriksen JH, Gerbes AL. Endothelin-1 and endothelin-3 in cirrhosis: relations to systemic and splanchnic haemodynamics. J Hepatol. 1995;23:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Tsai YT, Lin HC, Yang MC, Lee FY, Hou MC, Chen LS, Lee SD. Plasma endothelin levels in patients with cirrhosis and their relationships to the severity of cirrhosis and renal function. J Hepatol. 1995;23:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Martinet JP, Legault L, Cernacek P, Roy L, Dufresne MP, Spahr L, Fenyves D, Pomier-Layrargues G. Changes in plasma endothelin-1 and Big endothelin-1 induced by transjugular intrahepatic portosystemic shunts in patients with cirrhosis and refractory ascites. J Hepatol. 1996;25:700-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Bernardi M, Gulberg V, Colantoni A, Trevisani F, Gasbarrini A, Gerbes AL. Plasma endothelin-1 and -3 in cirrhosis: relationship with systemic hemodynamics, renal function and neurohumoral systems. J Hepatol. 1996;24:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Nagasue N, Dhar DK, Yamanoi A, Emi Y, Udagawa J, Yamamoto A, Tachibana M, Kubota H, Kohno H, Harada T. Production and release of endothelin-1 from the gut and spleen in portal hypertension due to cirrhosis. Hepatology. 2000;31:1107-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Rieder H, Ramadori G, Meyer zum Büschenfelde KH. Sinusoidal endothelial liver cells in vitro release endothelin--augmentation by transforming growth factor beta and Kupffer cell-conditioned media. Klin Wochenschr. 1991;69:387-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |