Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2254
Revised: December 23, 2003
Accepted: January 8, 2004
Published online: August 1, 2004
AIM: To explore the pathophysiological significance of delayed type hypersensitivity (DTH) reaction in mouse gastrointestinal tract induced by an allergen 2,4-dinitrochlorobenzene (DNCB).
METHODS: BALB/c mice were randomly divided into control and DTH1-6 groups. After sensitized by DNCB smeared on the abdominal skin, the mice were challenged with DNCB by gavage or enema. The weight, stool viscosity and hematochezia were observed and accumulated as disease active index (DAI) score; the gastrointestinal motility was represented by active charcoal propulsion rate; the colon pathological score was achieved by macropathology and HE staining of section prepared for microscopy; and the leukocyte migration inhibitory factor (LMIF) activity was determined by indirect capillary assay of the absorbance (A) of migrated leukocytes.
RESULTS: Active charcoal propulsion rates of small intestine in the DNCB gavages groups were significantly higher than that in the control group (P < 0.01). The DAI scores and pathological score in DNCB enema groups were also higher than that in the control group ( P < 0.05), and there were significant rises in LMIF activity in DNCB enema groups as compared with control groups (P < 0.01).
CONCLUSION: Mouse gastrointestinal DTH reaction could be induced by DNCB, which might facilitate the mechanism underlying the ulcerative colitis.
- Citation: Yu WG, Lin P, Pan H, Xiao L, Gong EC, Mei L. Pathophysiological significance of a reaction in mouse gastrointestinal tract associated with delayed-type hypersensitivity. World J Gastroenterol 2004; 10(15): 2254-2258
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2254.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2254
The gastrointestinal tract (GI) is not only an important organ in digestion and endocrine, but also the largest peripheral immunological organ in the body[1]. The secretory immunoglobulin A (s-IgA) and T cell are responsible for developing mucosa vaccines and producing oral tolerance[2,3]. Furthermore, the manifestation of T cell mediated reaction in GI tract was most often in the way of delayed-type hypersensitivity (DTH), thereby resulting in ulcerative colitis[4,5]. 2,4 -dinitrochlorobenzene (DNCB), a chemical compound of low molecular weight, could combine with tissue protein to function as a full antigen in activating T cell mediated DTH reaction, such as skin DTH[6] and colon inflammation/ulcer[5,7,8]. Gastrointestinal DTH (or colitis) induced by DNCB in rabbit, guinea pig and rat has been reported[5,7,8], but that in mice has not been reported. In this study, DNCB-induced DTH in mice GI tract were found, and its pathophysiological significance was discussed.
Healthy male BALB/c mice weighing 18-21 g (supplied by the Department of Experimental Animals, Peking University Health Science Center) were used. Animals were fed with a standard diet and allowed free access to water.
DNCB solution For DNCB sensitization, 330 mg DNCB (Beijing Chemical Reagent Company, Beijing, P.R. China) was dissolved in 10 mL of acetone-olive oil (1:1) vehicle. For DNCB gavage, 300 mg DNCB was first mixed with a minimum volume of polysorbate 80, and a minimum volume of ethanol was added until the mixture was completely dissolved, followed by the addition of olive oil to achieve a final DNCB concentration of 6.6 g/L. The ratios of polysorbate 80-ethanol-olive oil in this vehicle were 6.6%, 8.8% and 84.6%, respectively. Then the 6.6% solution was diluted to 1.3 g/L and 0.3 g/L DNCB solution. For DNCB enema, DNCB was dissolved in 600 mL/L ethanol to achieve 4 g/L, 2 g/L, and 1 g/L DNCB solution, separately. All these DNCB solutions were stored at 4 °C.
Activated charcoal suspension The suspension was made according to Qi et al[9] with a little modification. A total of 6 g activated charcoal (Tianjin 6th Chemical Reagent Company, Tianjin, China) and 2 g astragalin gum (Beijing Chemical Reagent Company, China) were dissolved in 50 mL of normal saline (NS) just before intragastric use.
RPMI 1640 One milliliter of RPMI 1640 culture medium contained 100 IU penicillin and 100 μg streptomycin, and then the pH was adjusted to 6.8-7.2.
Phytohemagglutinin (PHA) A 100 mg PHA was dissolved in 10 mL incomplete RPMI1640, and then sterilized and preserved at -20 °C.
Ammonium chloride solution (erythrocyte lytic fluid) A total of 1.03 g Tris and 3.735 g NH4Cl were dissolved in 500 mL distilled water.
Grouping Ninety-six BALB/c mice were randomly divided into 10 groups, including DTH1-6, DTH negative (DTH-) group and control group.
Sensitization On the first day of experiment, mouse fur on abdomen (2.5 cm diameter) was shaved. A total of 50 μL of 33g/L DNCB was smeared on the shaved skin once a day for 1 or 4 d. Mice in the DTH(-) and control groups were smeared with acetone-olive oil vehicle.
Gavage All the mice were fasted for 6 h before gavage. On the second day of experiment the mice in DTH1, DTH2 and DTH3 groups were gavaged with 0.8 mL of 0.3 g/L, 1.3/L and 6.6 g/L DNCB, respectively. The DTH(-) group was gavaged 1.3 g/L DNCB and the control group was gavaged polysorbate 80-ethanol-olive oil vehicle. On the third day, 0.8 mL of activated charcoal was gavaged to each mouse. Twenty minutes later, the mice were killed by decapitation for determination of gastrointestinal motility.
Enema On the 5 th day, a silica-gel tube with 10 mm diameter was inserted into the colons of the mice, its tip being 3-3.5 cm far from the anus. The mice in DTH4, DTH5 and DTH6 groups were intracolonically administered 1 g/L, 2 g/L and 4 g/L DNCB (2 μL/g) once a day for 4 d. DTH( -) group was administered 2 g/L DNCB and the control group was administered 600 mL/L ethanol vehicle.
Gastrointestinal motility After each mouse was killed, the whole small intestine was taken out, rinsed with NS quickly, and the intestinal wall was cut open longitudinally, laid and unfolded on a flat plate for estimation of the active charcoal migration distance (cm). The gastrointestinal motility was expressed by charcoal propulsion rate by using the formula: charcoal propulsion rate = migration distance of active charcoal / the distance from pylorus-duodenum junction to ileocecum×100%.
Body mass and stool By using disease activity index (DAI) score[10], the body mass and stool were scored as follows: Body mass: score 0-normal; score 1-1-5% lower than normal; score 2-6-10% lower than normal; score 3-11-15% lower than normal; and score 4-above 15% lower than normal. Stool viscosity: score 0-normal; score 2-fluffy; and score 4-diarrhea. Stool hemorrhage: score 0-normal; and score 2-apparent hemorrhage.
Pathological score On the 9 th d, all the mice received enema were killed by cervical dislocation. The colon was cut open longitudinally along the attachments of mesenteries and was first macropathologically observed. Then specimens were taken from inflammatory/ulcerative colon and were fixed in 40 g/L formaldehyde. Paraffin-embedded 5-μm thick section was made for HE staining. The macropathological score and microscopy score were obtained by adopting Dr. Murano’s method[10] with slight modification as follows: Macropathological score: score 0-no cementation (colon was easily detached from other tissues) and no inflammation in colon; score 1-moderate cementation or local congestion; score 2-severe cementation with one ulcer (< 1 cm); score 3- more than one ulcer (< 1 cm) with inflammation; and score 4-more than one ulcer (> 1 cm) with inflammation.
Microscopy: score 0- normal colon mucosa or slight congestion; score 1-mucosa hyperemia, infiltration of chronic inflammatory cells and decrease of the number of goblet cell in colon mucosa; score 2-mucosa hyperemia, infiltration of chronic inflammatory cells and local superficial erosion; and score 3-atrophic change with ulcer and serious infiltration of chronic inflammatory cells in colon mucosa.
LMIF preparation By following our previous method[11], the spleen lymphocytes (2 × 106/mL) of mice received enema were cultured in PHA (60 μg/mL) for 72 h. Then the supernatant containing LMIF component was collected by centrifugation (1 500 r/min, 15 min), followed by lyophilization. The lyophilized powder was stored at -20 °C. Working solution (LMIF solution) was prepared by dissolving the lyophilized powder in RPMI-1640 to one third of its original volume before using.
Preparation of peripheral leukocytes A suspension of indicator cells (migrated leukocytes) was prepared from the anti-coagulated whole blood of guinea pigs. Three percent gelatin of 1/3 volume of the blood was added to the blood before culturing the sample at 37 °C for 30 min. The upper layer rich in leukocytes was taken out and centrifuged (1 000 r/min) for 10 min. Then the sediment was washed twice with Hanks’ solution free of Mg2+ and Ca2+, and again centrifuged (1 000 r/min) for 10 min. Finally, the sediment was used as the indicator cell and was adjusted to a concentration of (1.6 - 1.8) × 107 cells/mL RPMI 1640.
Detection of leukocyte absorbance By following our previous work[11] with a little improvement, the leukocyte was injected into a capillary tube of 80 μL volume. After heat-sealing one end of the tube and spinning at 1 000 r/min for 10 min, the tubes were cut at the liquid-cell interface. The portion containing the cells was placed inside each chamber of a 24-well plate filled with 210 μL LMIF solution for culture. Twelve hours later, 200 μL solution was taken out from each chamber and was put in an well of a 96-well plate, followed by an addition of 20 μL methylthiazoletetrazolium, MTT) was added into each well before the plate was incubated at 37 °C for 4 h. Then the supernatant was removed, 200 μL of DMSO was added into each well and the plate was shaken for 10 min. The absorbance (A value) of the migration cells was determined in Micro plate Reader at a wavelength of 570 nm. The average of A values of 3 wells was regarded as a mean A value. The above processes were repeated for 9 times.
Data were expressed as mean ± SE. Analysis of variance (ANOVA) and student’s t test were used for comparison among the groups and between paired data. P < 0.05 was considered to be statistically significant.
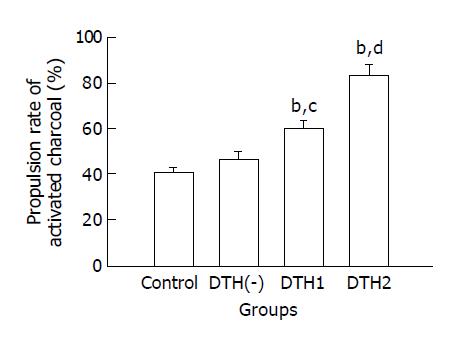
Active charcoal propulsion rates of small intestine in the DTH1 (0.3 g/L DNCB) and DTH 2 (1.3 g/L DNCB) groups were significantly higher than that in the control group (P < 0.01) and DTH(-) groups (P < 0.05 or P < 0.01), whereas there was no significant difference between the control group and DTH(-) group (Figure 1). All the mice in DTH3 group died after the 0.66% DNCB gavage.
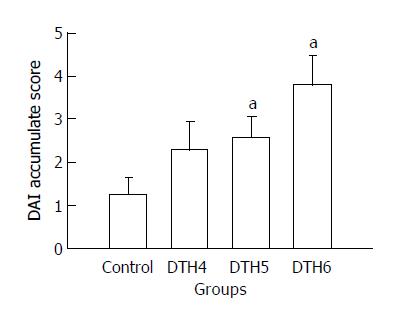
Diarrhea was first found in the mice 24 h after DNCB enema, and weight loss was found 3 d later. Serious weight loss and obvious diarrhea were seen in the 4 g/L DNCB group, 24% of whom died. DAI scores are shown in Figure 2.
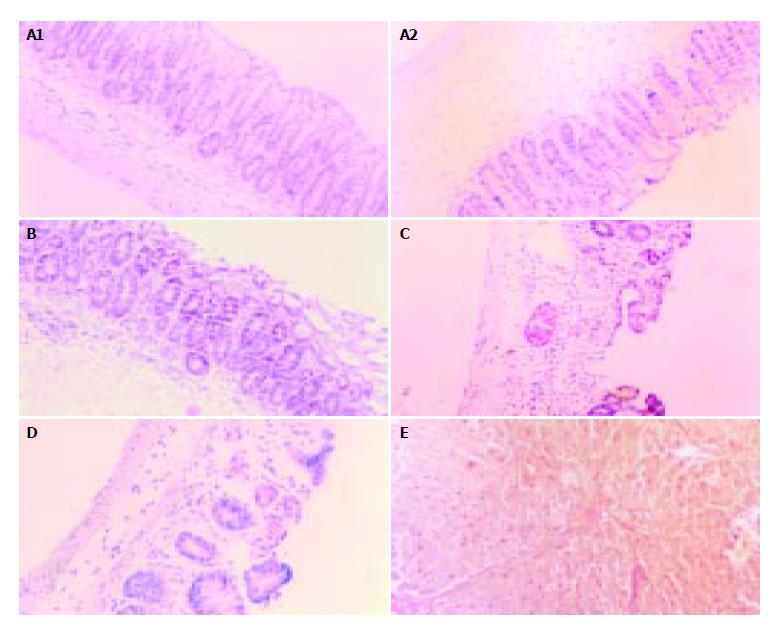
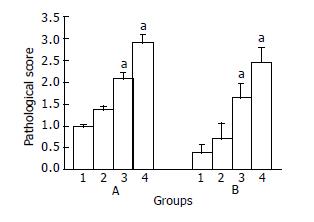
Pathologically, the control and DTH(-) groups had normal histological structures and glands, no ulcer was found except for occasional slight mucosa congestion (Figure 3A). In DTH4 (1 g/L DNCB) group, there was slight colic edema, mucosa congestion, infiltration of lymphocytes and a decrease in the number of glands and goblet cells (Figure 3B). In DTH5 (2 g/L DNCB) group, intestinal adhesion and flatulence were found. A more disturbing array of glands, local erosion, dramatic decrease in goblet cells and diffuse inflammatory cellular infiltration were found (Figure 3C). In DTH6 (4 g/L DNCB) group, more extensive colic cementation, expansion of the proximal intestinal cavity, some white exudates, mucosa congestion, necrosis and multiple ulcers were found. Under the microscope, mucosa atrophy, decrease of glands and disturbance of tissue structure were observed (Figure 3D), moreover, erosion, hemorrhage, necrosis as well as deeper/extensive ulcers were easily seen (Figure 3E). The pathological score in each group is shown in Figure 4.
With the increased DNCB doses in enema, which exacerbated colonic tissue damage, we could see an increase of the LMIF activity (A value decreased). The LMIF activity was significantly increased in the DTH5 and DTH6 groups as compared with the control group (P < 0.01, student’s t-test) (Table 1). There were also significant differences in LMIF activity (P < 0.01) among DTH4, DTH5 and DTH6 groups (ANOVA).
For the first time, here, we reported DTH response of mice GI tract by intra-gastrointestinal administration of DNCB. The evidence of gastrointestinal DTH response were as follows: DNCB-sensitized mouse showed increased GI motility, diarrhea, hematochezia and colitis after DNCB challenge; and the activity of LMIF released from sensitized T- lymphocyte was significantly increased after DNCB enema. The mechanism of the DTH phenomenon might be the same as that of the ear skin DTH reaction in our previous study[6].
What is the pathophysiological significance of GI DTH reaction Early in the 1960’s, Bicks et al[4] found that inflammation and ulcer could be induced by DNCB enema in guinea pigs, which he thought as being a cellular immune induced by DNCB that resulted in a DTH reaction in GI tract. Later, several other researchers explored the relations between DNCB-activited cellular immune and GI disorder, but they either indirectly inferred the possibility of GI being influenced by DTH skin tests[12] or only focused on the pathological relation of DNCB-colitis with human ulcerative colitis (UC)[5,7,8]. Comparing with previous DNCB-colitis in rat, guinea pig and rabbit by other researchers, our work here not only successfully created DNCB-colitis model in mouse, but also found a more convenient and more sensitive animal model for DTH study[13]. In our study, we proved, using the specific DTH index of LMIF[14,15], that DNCB could induce DTH in mouse GI tract, and this kind of DTH might be the essence of UC.
MIF is one of the cytokines released from sensitized T cell after subjecting the allergen again[14,15]. According to the target cell, LMIF can be classified as LMIF and MMIF[16]. There was a dramatic positive correlation between LMIF activity and the gravity of DTH response[17]. We found that the ConA-stimulated lymphocytes from mesenteric lymph node of DNCB-treated mouse showed higher LMIF activity than the control, and LMIF activity increased as DNCB concentration increased (Table 1), whereas in DTH(-) group, neither higher LMIF activity nor colon inflammation/ulcer was found, indicating a possible relations among GI DTH response, LMIF activity and UC. Secondly, we know that the main clinical manifestations of UC are diarrhea and pus hematochezia, which result from an irritated intestinal peristaltic and inflectional ulcer[18,19]. Our results were in accordance with the clinical manifestations of UC (Figures 1-4), indicating that colitis in mouse caused by DNCB was basically similar to human UC in pathology. Finally, two representatives but separate works by Bartnik et al[20] and Murakami et al[21] support the hypothesis that the essence of UC is gastrointestinal DTH response. Bartnik et al[20] reported that patients with severe or moderate ulcerative colitis showed LMIF release, which was significantly greater than that observed in patients with other large bowel diseases; and Murakami et al[21] reported that the number of MIF expressing cells increased at the colonic mucosa in patients with ulcerative colitis, and MIF induced significant levels of IL-1 and IL-8 in monocytes and dendrite cells in UC patients, indicating a role of MIF in the induction and/or perpetuation of the inflammatory environment in UC[21]. Comparing with all the other cytokines reported in UC[22], we think that the distinctive (unique) significance of LMIF in UC and its pivotal role in connecting DTH with UC have been showed by Bartnik and Murakami.
To sum up, we have enough reasons to conclude that the GI DTH in mouse may provide not only experimental models of human UC, but also insight into pathogenic mechanisms of the UC, an inflammatory bowel disease (IBD) of unknown etiology.
Edited by kumar M and Xu FM
| 1. | Takahashi I, Kiyono H. Gut as the largest immunologic tissue. JPEN J Parenter Enteral Nutr. 1999;23:S7-12. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Czerkinsky C, Anjuere F, McGhee JR, George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF, Pierrefite-Carle V, Rask C. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev. 1999;170:197-222. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Galliaerde V, Desvignes C, Peyron E, Kaiserlian D. Oral tolerance to haptens: intestinal epithelial cells from 2,4-dinitrochlorobenzene-fed mice inhibit hapten-specific T cell activation in vitro. Eur J Immunol. 1995;25:1385-1390. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | BICKS RO, ROSENBERG EW. A CHRONIC DELAYED HYPERSENSITIVITY REACTION IN THE GUINEA PIG COLON. Gastroenterology. 1964;46:543-549. [PubMed] [Cited in This Article: ] |
| 5. | Rabin BS, Rogers SJ. A cell-mediated immune model of inflammatory bowel disease in the rabbit. Gastroenterology. 1978;75:29-33. [PubMed] [Cited in This Article: ] |
| 6. | Mei L, Li L, Li Y, Deng Y, Sun C, Ding G, Fan S. Conditioned immunosuppressive effect of cyclophosphamide on delayed-type hypersensitivity response and a preliminary analysis of its mechanism. Neuroimmunomodulation. 2000;8:45-50. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Glick ME, Falchuk ZM. Dinitrochlorobenzene-induced colitis in the guinea-pig: studies of colonic lamina propria lymphocytes. Gut. 1981;22:120-125. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Zhang YB, Zou YH, Lian ZC, Chen WQ. Experimental model of ulcerative colitis in rat and its abnormality of colonic electricity. Laboratory Animal Science Administration. 2002;19:5-7. [Cited in This Article: ] |
| 9. | Qi HB, Luo JY, Liu X. Effect of enterokinetic prucalopride on intestinal motility in fast rats. World J Gastroenterol. 2003;9:2065-2067. [PubMed] [Cited in This Article: ] |
| 10. | Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51-58. [DOI] [Cited in This Article: ] |
| 11. | Mei L, Li LQ, Fan SG, Ding GF. An assay of leukocyte migration inhibitory factor (LMIF) and the conditioned sup-pression effect on LMIF. Chin J Microbiol Immunol. 1998;18:474-478. [Cited in This Article: ] |
| 12. | Shell-Duncan B, Wood JW. The evaluation of delayed-type hypersensitivity responsiveness and nutritional status as predictors of gastro-intestinal and acute respiratory infection: a prospective field study among traditional nomadic Kenyan children. J Trop Pediatr. 1997;43:25-32. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Gold D. Delayed-type hypersensitivity to Entamoeba histolytica in mice and hamsters: a comparison. Parasitol Res. 1989;75:335-342. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80-82. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Wolberg WH, Goelzer ML. In vitro assay of cell mediated immunity in human cancer: definition of leukocyte migration inhibitory factor. Nature. 1971;229:632-634. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Matsui Y, Oshima S. Migration inhibition and stimulation factors produced from peripheral blood lymphocyte cultures of sensitised guinea pigs. Asian Pac J Allergy Immunol. 1985;3:151-155. [PubMed] [Cited in This Article: ] |
| 17. | Malorny U, Goebeler M, Gutwald J, Roth J, Sorg C. Differences in migration inhibitory factor production by C57Bl/6 and BALB/c mice in allergic and irritant contact dermatitis. Int Arch Allergy Appl Immunol. 1990;92:356-360. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Soffer EE. Diarrhea and malabsorption In: Stoller JK, Ahmad M, Longworth DL, eds. The cleveland clinic intensive review of internal medicine. New York Lippincott Williams Wilkins. 2000;730-732. [Cited in This Article: ] |
| 19. | Stenson WF. Inflammatory bowel disease In: Yamada T, Apers DH, Laine L, Owyang C, Powell DW, eds. Textbook of gastroenterology. New York Lippincott Williams Wilkins. 1999;1782-1783. [Cited in This Article: ] |
| 20. | Bartnik W, ReMine SG, Shorter RG. Leukocyte migration inhibitory factor (LMIF) release by human colonic lymphocytes. Arch Immunol Ther Exp (Warsz). 1981;29:397-405. [PubMed] [Cited in This Article: ] |
| 21. | Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Macrophage migration inhibitory factor activates antigen-presenting dendritic cells and induces inflammatory cytokines in ulcerative colitis. Clin Exp Immunol. 2002;128:504-510. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Zhou T, Lin P, Pan H, Mei L. Ulcerative colitis: a review in its pathogenesis and immune mechanisms. Shijie Huaren Xiaohua Zazhi. 2003;11:1782-1786. [Cited in This Article: ] |