Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1907
Revised: February 2, 2004
Accepted: February 9, 2004
Published online: July 1, 2004
AIM: To prospectively assess the sensitivity, specificity and time to positivity of theUltra-rapid urease test (URUT) for Helicobacter pylori (H pylori ), and compare the results of one with those of two biopsies.
METHODS: Five antral biopsies were taken in consecutive patients undergoing upper endoscopy: one and two biopsies for URUT, and one each for H pylori culture and histology. URUT was read at 1, 5, 10, 20 and 30 min, 1, 2, 3 and 24 h after biopsy insertion into the reagent. A positive histology and/or culture was used as positive reference ”gold standards”.
RESULTS: URUT was more sensitive for detecting H pylori with two biopsies rather than one, at all time points up to 120 min. The sensitivity improved from 3.6% to 82.1% for one biopsy and 10.7% to 85.7% for two biopsies from 1 to 120 min. The sensitivity reached 96.4% at 24 h for both, but the specificity reduced from 100% to 96% and 92% for one and two biopsies, respectively.
CONCLUSION: Development of a positive URUT result is hastened by doubling the number of gastric biopsies. We recommend taking two instead of one biopsy to achieve an earlier positive URUT result so that H pylori eradication therapy can be initiated before patient is discharged from the endoscopy suite.
-
Citation: Lim LL, Ho KY, Ho B, Salto-Tellez M. Effect of biopsies on sensitivity and specificity of ultra-rapid urease test for detection of
Helicobacter pylori infection: A prospective evaluation. World J Gastroenterol 2004; 10(13): 1907-1910 - URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1907
The biopsy urease test was introduced as a simple and convenient method for diagnosing Helicobacter pylori (H pylori) infection. The biopsy urease test is based on the presence of large amounts of preformed urease enzymes in H pylori. The breakdown of urea by urease produces a high local concentration of ammonia, which raises the gastric pH. A phenol indicator that changes the color from yellow at pH6.8 to magenta at pH8.4 can detect this pH alteration. The color change with the introduction of the gastric biopsy is an indication for the presence of H pylori.
Despite many advances on the study of H pylori, the use of the biopsy urease test still remains invaluable in the diagnosis and management of gastroduodenal disease[1,2]. The rapid urease test is able to offer a sensitivity of 80%-99% and a specificity of 92%-100%[3-5] in untreated patients when compared with histology as the gold standard in the diagnosis of H pylori infection.
Since McNulty and Wise first described the biopsy urease test in 1985[6], several modifications of this test have been developed and validated. A test kit (CLOtest, Delta West Limited, W Australia) is commercially available but its costs and the time taken to positive results, which may be up to 24 h, limits its usefulness. The detection rate of H pylori at 20 min was 75% and 92% at 3 h and 98% at 24 h[7]. The length of time it takes for CLOtest still poses a hindrance to those clinicians wishing to treat infected patients while they are still in the endoscopy room.
The ultra-rapid urease test (URUT), as described by Arvind[8] is reported to overcome the latter shortcoming by enabling most positive tests to be apparent even before the end of the endoscopy[8].
We had previously reported the sensitivity, specificity, and the time to positivity for the URUT[9]. We found that better sensitivity could be obtained if the test continued to be read over a 24-h period although this was achieved at the expense of an increase in the number of false positive cases. We used only one antral biopsy for the URUT. In the CLOtest, the use of two biopsy specimens has been shown to hasten the time to a positive reaction[10]. Taking two specimens also may theoretically improve the sensitivity and specificity of the test.
The aim of this study was to assess prospectively the sensitivity, specificity and time to positivity for the URUT and compare the results of one biopsy and those of two biopsies in patients undergoing upper endoscopy.
From September 1999 to June 2000, 53 consecutive patients who were given routine diagnostic upper gastrointestinal endoscopic examinations by one of the authors (KYH) and who had not been exposed to antibiotics, proton pump inhibitor or bismuth compounds within the past four weeks, and in whom gastric antral biopsies were clinically indicated, were prospectively studied. Patients who had been previously treated for H pylori infection and those with an abnormal coagulation profile were excluded from the study.
After an overnight or six-hour fast, each patient underwent esophagogastroduodenoscopy, during which five antral biopsies were taken from within 2 cm of the pylorus using sterilised biopsy forceps (Olympus 16K; Olympus Corp., Tokyo, Japan). Three of the biopsies were used for URUT wherein the tests were done using one and two of the biopsies, respectively. The remaining two biopsies were sent for culture and histologic examination for H pylori. Biopsy specimens for the urease test and culture were taken before those used for histologic examination to avoid contamination with formalin. All patients gave informed consent before endoscopy. The study was conducted in accordance with the provisions of the Declaration of Helsinki and was approved by the Hospital Research & Ethics Committee.
The ultra-rapid urease test kit is composed of a capped polypropylene tube containing 0.5 mL aliquot of 100 g/L unbuffered solution of urea in deionised water (pH6.8). The solution contains two drops of 10 g/L phenol red, which acts as the indicator[11]. The biopsy specimens for the URUT were removed from the biopsy forceps with a sterile toothpick and placed immediately into the polypropylene tube. Particular care was taken not to shake the tube after placing the biopsy into it so that a rapid positive result could be achieved[12]. The reagent was prepared in large batches, frozen, and was ready for thawing just before use. A positive test result was indicated when there was a color change in the medium surrounding the biopsy from yellow to magenta. The test tube was left at room temperature and examined by experienced observers without knowing the clinical details at pre-determined intervals over 24 h. Convenient times chosen were 1, 5, 10, 20, 30 min and 1, 2, 3 and 24 h after insertion of the biopsy specimen into the urease test reagent.
The gastric biopsies for histologic assessment were fixed in 40 g/L neutral buffered formaldehyde paraffin processed and stained with haematoxylin and eosin (H&E) in the normal way. Experienced pathologists without knowledge of the clinical data and URUT results assessed the presence or absence of H pylori by examining 3 sets of tissue levels within 12 consecutive sections. Histologic diagnosis of H pylori infection with H&E stain was compared with the use of Giemsa[13,14] and thus the Giemsa stain was used only when a few organisms were identified with H&E stain, always confirming the original diagnostic impression.
For H pylori culture, gastric biopsies were smeared on pre-reduced chocolate blood agar plates and incubated in a humidified carbon dioxide incubator at 37 °C with 50 mL/L carbon dioxide for 3-4 d. The identity of any colonies grown was confirmed using Gram’s stain and biochemical tests. Experienced microbiologists without knowledge of the clinical data and URUT results assessed the presence or absence of H pylori[15,16].
Only patients with both culture and histology results were analyzed. Results from the URUT were compared with those from culture and histology of biopsy specimens obtained from the same patient. H pylori was considered as positive if either culture or histology demonstrated H pylori, and negative if H pylori was not detected in both culture and histology. Based on these results, the sensitivity and specificity of the URUT at various time points were determined. For this study, sensitivity was defined as the frequency of a positive URUT in patients with either gastric histology or culture positive for H pylori. Specificity was the frequency of a negative test in patients with both negative histology and culture for H pylori.
Of the 53 patients included in this study, 39 (74%) were Chinese, 7 (13%) were Malays, 6 (11%) were Indians and 1 (2%) was of another race. There were 29 (55%) males and 24 (45%) females. Their ages ranged from 23 to 85 (median, 52) years. The indications for the endoscopy included abdominal pain in 33 patients (62.2%), anemia or drop in hemoglobin in 6 (11.3%), atypical chest pain in 5 (9.5%), gastric ulcer follow-up in 1 (1.9%), and other symptoms in 8 (15.1%). The findings during esophagogastroduodenoscopy were esophagitis in 15 (28%), duodenal ulcers in 14 (26%), gastric ulcers in 8 (15%), gastritis in 8 (15%), duodenitis in 3 (6%), and others in 5 (10%).
H pylori was positive in 28 of the 53 patients (53%), in whom either the histology or culture demonstrated the organism. Twenty-four patients were H pylori positive in histology and 28 patients were culture positive.
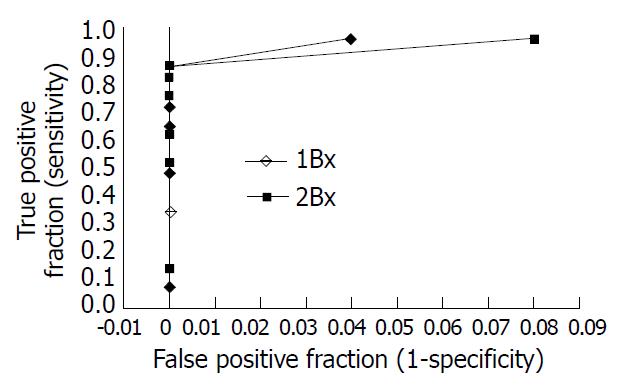
The results of sensitivity and specificity of URUT at various time points are shown in Table 1. URUT was more sensitive for detecting H pylori when two biopsies rather than one were used, at all time points up to 120 min. At 1 min, the sensitivity was 3.6% for one biopsy and 10.7% for two biopsies. At 5 min, the sensitivities improved to 32.1% and 50% respectively. At 60 min, the sensitivity was 82.1% for one biopsy and 85.7% for two biopsies. Thereafter there was still some increase in sensitivity with either one or two biopsies, reaching 96.4% at 24 h in each case, but with a reduction in the specificity from 100% to 96% and 92%, for one and two biopsies respectively. Using the receiver operating characteristic curve (Figure 1), the optimal times to read the test appeared to be 1 h when two biopsies were used and 3 h when only a single biopsy was used, and at these time points, the optimal combination of sensitivity and specificity was obtained.
| 1 m | 5 m | 10 m | 20 m | 30 m | 60 m | 2 h | 3 h | 24 h | ||
| True positive | 1 Bx | 1 | 9 | 13 | 18 | 20 | 23 | 23 | 24 | 24 |
| 2 Bx | 3 | 14 | 17 | 21 | 23 | 24 | 24 | 24 | 27 | |
| True negative | 1 Bx | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 24p |
| 2 Bx | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 23 | |
| False positive | 1 Bx | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 2 Bx | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| False negative | 1 Bx | 27 | 19 | 15 | 10 | 8 | 5 | 5 | 4 | 1 |
| 2 Bx | 25 | 14 | 11 | 7 | 5 | 4 | 4 | 4 | 1 | |
| Sensitivity (%) | 1 Bx | 3.6 | 32.1 | 46.4 | 64.3 | 71.4 | 82.1 | 82.1 | 85.7 | 96.4 |
| 2 Bx | 10.7 | 50 | 60.7 | 75 | 82.1 | 85.7 | 85.7 | 85.7 | 96.4 | |
| Specificity (%) | 1 Bx | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 96 |
| 2 Bx | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 92 | |
| PPV (%) | 1 Bx | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 96.4 |
| 2 Bx | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 96.4 | |
| NPV (%) | 1 Bx | 48.1 | 55.6 | 62.5 | 71.4 | 75.8 | 83.3 | 83.3 | 86.2 | 96 |
| 2 Bx | 50 | 64.1 | 69.4 | 78.1 | 83.3 | 86.2 | 86.2 | 86.2 | 95.8 | |
The ultra-rapid urease test, as described by Arvind[8] is reported to overcome the shortcoming of the CLOtest which takes a while to become positive. Using this home-made URUT preparation, we had found the test to be highly specific but not very sensitive when interpreted at one minute[9]. However, if the test continued to be read, the sensitivity improved to 87% at 24 h. This was however achieved at the expense of a longer reading time and a reduction in specificity to 90% at 24 h[9]. The development of false positives with delayed reading of the urease tests has previously been noted and attributed to other urease-producing bacteria, or to relatively small numbers of H pylori, which are not identified histologically, i.e., false-negative histology[17].
We previously used only one antral biopsy for the ultra-rapid urease test[9]. We hypothesize that if more gastric tissue was sampled, a positive test result might appear earlier, thus improving the test sensitivity without compromising its specificity. The present study confirmed this hypothesis. The URUT was more sensitive in detecting H pylori when two biopsies instead of one, were used at all time points up to 60 min. In this study a receiver operating characteristic curve analysis was used to select the optimal time for reading the test. The 1-h reading was found to give the optimal sensitivity/specificity combination when two biopsies were used, this does not automatically ensure that it is the best time to read the test. In many centers in the Asia Pacific region, the URUT is used as an initial screening test, with an additional sample taken for histology, which is sent only if the URUT is negative and when there is a strong clinical suspicion of H pylori infection. This approach is of great benefit to avoid the additional cost of the histology. Despite the fact that the H pylori infection is a chronic disease, the necessity to treat it soon after endoscopy is desirable to minimize the cost as well as the inconvenience of a second clinic visit. If this is the practice, then the most appropriate time point at which to read the test is 30 min when there is maximal specificity (as false positives will not be checked by histology) and minimal gain in sensitivity by reading the test beyond this time. Most importantly, reading the test at 30 min allows the physician to prescribe H pylori eradication treatment to the patient before discharging him or her from the endoscopy suite.
We are unable to explain the initial low sensitivity of the URUT test results found in the this study when compared with the original study[9]. One possible explanation is the reduced size of the biopsy specimens using smaller biopsy forceps as compared with the original study. However, the discrepancy is unlikely to be caused by the difference in the study populations, the incubation temperature of the biopsy specimens, and the volume and concentration of the phenol red used to make the test reagents since these parameters were similar to our previous study[9]. The initially low test sensitivity might not be a major problem as test sensitivity difference between one and two biopsies, and not the initial test sensitivity was the primary concern of this study. The conclusions reached by this study therefore, should remain valid.
There may be additional yield in taking biopsy specimens from different sites of the stomach due to the differences in the geographical distribution of H pylori, for example from the gastric body, in addition to the antral biopsies. However, we do not believe the addition of these biopsies would add significantly to the sensitivity of the tests. As part of another protocol (personal communication, KYH), two antral and two body biopsies were taken from 115 consecutive patients, and the specimens stained with H&E to assess the presence or absence of H pylori. Of a total of 66 histology positives, only three (4.5%) were correctly identified by the body biopsies while negative in the antral biopsies. Thus body biopsies contributed to an increased yield of only 4.5% in identifying histology positives. Previous other studies to determine the topographic distribution and density of H pylori to provide the maximum yield and to reduce the fallacious results due to sampling error have also concluded that biopsies taken from the antrum was sufficient[18-20].
Increasing the number of biopsies to more than two biopsies from the antrum, that is three or even four antral biopsy specimens, may increase the sensitivity given that this probably increases the H pylori load and therefore the amount of urease. However, this will prolong the endoscopy time and add inconvenience to the patient.
In our study, H pylori positivity was 53% based on either culture or histology result, which is in agreement with the local background H pylori colonization rate from our previously published data[9,21]. There were 24 patients who were H pylori positive on histology and 28 patients who were culture positive. This difference may reflect the difference in H pylori density. In this case, culture appears more sensitive than histology in identifying H pylori.
In conclusion, this study showed that the development of a positive URUT result could be hastened by doubling the gastric tissue samples. We recommend to take two biopsies instead of one for the URUT to achieve an earlier positive test result so that appropriate H pylori eradication therapy can be prescribed before the patient is discharged from the endoscopy suite.
Edited by Ma JY Proofread by Xu FM
| 1. | Basset C, Holton J, Ricci C, Gatta L, Tampieri A, Perna F, Miglioli M, Vaira D. Review article: diagnosis and treatment of Helicobacter: a 2002 updated review. Aliment Pharmacol Ther. 2003;17 Suppl 2:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Versalovic J. Helicobacter pylori. Pathology and diagnostic strategies. Am J Clin Pathol. 2003;119:403-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Wong BC, Wong WM, Wang WH, Tang VS, Young J, Lai KC, Yuen ST, Leung SY, Hu WH, Chan CK. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001;15:505-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Chen YK, Godil A, Wat PJ. Comparison of two rapid urease tests for detection of Helicobacter pylori infection. Dig Dis Sci. 1998;43:1636-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Viiala CH, Windsor HM, Forbes GM, Chairman SO, Marshall BJ, Mollison LC. Evaluation of a new formulation CLOtest. J Gastroenterol Hepatol. 2002;17:127-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | McNulty CA, Wise R. Rapid diagnosis of Campylobacter-associated gastritis. Lancet. 1985;1:1443-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Marshall BJ, Warren JR, Francis GJ, Langton SR, Goodwin CS, Blincow ED. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987;82:200-210. [PubMed] [Cited in This Article: ] |
| 8. | Arvind AS, Cook RS, Tabaqchali S, Farthing MJ. One-minute endoscopy room test for Campylobacter pylori. Lancet. 1988;1:704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Ho KY, Kang JY, Lim TP, Yeoh KG, Wee A. The effect of test duration on the sensitivity and specificity of ultra-rapid urease test for the detection of Helicobacter pylori infection. Aust N Z J Med. 1998;28:615-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Laine L, Chun D, Stein C, El-Beblawi I, Sharma V, Chandrasoma P. The influence of size or number of biopsies on rapid urease test results: a prospective evaluation. Gastrointest Endosc. 1996;43:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Thillainayagam AV, Arvind AS, Cook RS, Harrison IG, Tabaqchali S, Farthing MJ. Diagnostic efficiency of an ultrarapid endoscopy room test for Helicobacter pylori. Gut. 1991;32:467-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Katelaris PH, Lowe DG, Norbu P, Farthing MJ. Field evaluation of a rapid, simple and inexpensive urease test for the detection of Helicobacter pylori. J Gastroenterol Hepatol. 1992;7:569-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kang JY, Wee A, Math MV, Guan R, Tay HH, Yap I, Sutherland IH. Helicobacter pylori and gastritis in patients with peptic ulcer and non-ulcer dyspepsia: ethnic differences in Singapore. Gut. 1990;31:850-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Kang JY, Ho KY, Yeoh KG, Guan R, Wee A, Lee E, Lye WC, Leong SO, Tan CC. Peptic ulcer and gastritis in uraemia, with particular reference to the effect of Helicobacter pylori infection. J Gastroenterol Hepatol. 1999;14:771-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Hua J, Ng HC, Yeoh KG, Ho KY, Ho B. Characterization of clinical isolates of Helicobacter pylori in Singapore. Microbios. 1998;94:71-81. [PubMed] [Cited in This Article: ] |
| 16. | Hua JS, Zheng PY, Fong TK, Khin MM, Bow H. Helicobacter pylori acquistion of metronidazole resistance by natural transformation in vitro. World J Gastroenterol. 1998;4:385-387. [PubMed] [Cited in This Article: ] |
| 17. | Laine L, Estrada R, Lewin DN, Cohen H. The influence of warming on rapid urease test results: a prospective evaluation. Gastrointest Endosc. 1996;44:429-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Misra V, Misra S, Dwivedi M, Singh UP, Bhargava V, Gupta SC. A topographic study of Helicobacter pylori density, distribution and associated gastritis. J Gastroenterol Hepatol. 2000;15:737-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Vassallo J, Hale R, Ahluwalia NK. CLO vs histology: optimal numbers and site of gastric biopsies to diagnose Helicobacter pylori. Eur J Gastroenterol Hepatol. 2001;13:387-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Kalantar J, Xia HHX, Ma Wyatt J, Rose D, Talley NJ. Determina-tion of optimal biopsy sites for detection of H pylori in patients treated for not treated with antibiotic and anti-secretory drugs. Gastroenterology. 1997;112:A165. [Cited in This Article: ] |
| 21. | Kang JY, Yeoh KG, Ho KY, Guan R, Lim TP, Quak SH, Wee A, Teo D, Ong YW. Racial differences in Helicobacter pylori seroprevalence in Singapore: correlation with differences in peptic ulcer frequency. J Gastroenterol Hepatol. 1997;12:655-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |