Copyright
©The Author(s) 2004.
World J Gastroenterol. Jun 15, 2004; 10(12): 1699-1708
Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1699
Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1699
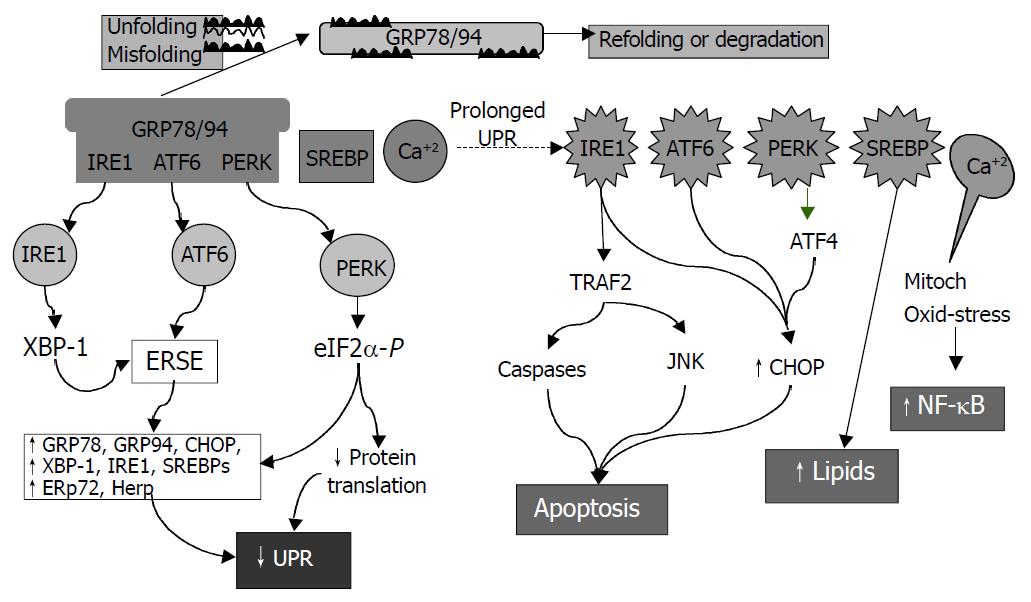
Figure 3 Consequences of endoplasmic reticulum (ER) stress response.
In the early phase, unfolded proteins cause dissociation of chaperones such as Bip/GRP78 from ER resident kinases-IRE1 and PERK and transcription factor-ATF6. Activated PERK phos-phorylates eIF2 resulting in translational attenuation. Activated IRE1 and ATF6 up-regulate genes encoding ER chaperone pro-teins such as GRP78/94 leading to increased protein-folding capacity. Overall, the unfolded protein response (UPR) goes down. In the late phase, IRE1 interacts with TRAF2 (tumor necrosis factor receptor associated factor 2) which activates caspases and JNK (cJUN NH2-terminal kinase) leads to apoptosis. ATF6 and PERK upregulate CHOP (C/EBP homologous protein) promoting cell death. SREBP upregulates lipid synthesis. Prolonged UPR leads to Ca2+ release from ER causing production of reactive oxygen intermediates which may lead to activation of NF-κB.
- Citation: Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol 2004; 10(12): 1699-1708
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1699.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1699