Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1755
Revised: December 9, 2003
Accepted: December 16, 2003
Published online: June 15, 2004
AIM: To demonstrate the effect of lactose as an inducer on expression of the recombinant proteins encoded by Helicobacter pylori ureB and hpaA, and Escherichia coli LTB and LTKA63 genes and to determine the optimal expression parameters.
METHODS: By using SDS-PAGE and BIO-RAD gel image analysis system, the outputs of the target recombinant proteins expressed by pET32a-ureB-E.coliBL21, pET32a-hpaA-E.coliBL21, pET32a-LTKA63-E.coliBL21 and pET32a-LTB-E.coliBL21 were measured when using lactose as inducer at different dosages, original bacterial concentrations, various inducing temperatures and times. The results of the target protein expression induced by lactose were compared to those by isopropyl-β-D-thiogalactoside (IPTG). The proteins were expressed in E.coli.
RESULTS: Lactose showed higher efficiency of inducing the expression of rHpaA, rUreB, rLTB and rLTKA63 than IPTG. The expression outputs of the target recombinant proteins induced at 37 °C were remarkably higher than those at 28 °C. Other optimal expression parameters for the original bacterial concentrations, dosages of lactose and inducing time were 0.8, 50 g/L and 4 h for rHpaA; 0.8, 100 g/L and 4 h for rLTKA63; 1.2, 100 g/L and 5 h for both rUreB and rLTB, respectively.
CONCLUSION: Lactose, a sugar with non-toxicity and low cost, is able to induce the recombinant genes to express the target proteins with higher efficiency than IPTG. The results in this study establish a beneficial foundation for industrial production of H pylori genetic engineering vaccine.
-
Citation: Yan J, Zhao SF, Mao YF, Luo YH. Effects of lactose as an inducer on expression of
Helicobacter pylori rUreB and rHpaA, andEscherichia coli rLTKA63 and rLTB. World J Gastroenterol 2004; 10(12): 1755-1758 - URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1755.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1755
In China, chronic gastritis and peptic ulcer are two most common gastric diseases[1], and gastric cancer is one of the malignant tumors with high mortalities and morbidities.
Helicobacter pylori (H pylori), a microaerophilic, spiral and Gram-negative bacterium, is recognized as a human-specific gastric pathogen that colonizes the stomachs of at least half of the world’s populations. Most infected individuals are asymptomatic. However, in some subjects, H pylori infection causes acute, chronic gastritis and peptic ulceration[2-4], and acts as a high risk factor on development of gastric adenocarcinoma[5-10], and mucosa-associated lymphoid tissue (MALT) lymphoma[10-13].
It is generally considered inoculation of H pylori vaccine is a most efficient measure for prevention and control of H pylori infection[14]. However, high nutrition requirements, poor growth for a long time, easy contamination during the cultivation and difficulty of bacterial strain conservation make whole cell vaccine of H pylori impracticable. Genetic engineering vaccine seems to be a possible pathway for developing H pylori vaccine.
Isopropyl-β-D-thiogalactoside (IPTG), a highly stable and effective inducer on T7lac promoter for target recombinant protein expression, is widely used in laboratories. However, IPTG is a reagent with potential toxicity and high cost[15], which limited it as a practical inducer for industrial production of genetic engineering vaccines. It was reported that lactose, a common disaccharide, is also able to induce T7lac promoter after it is transformed into allolactose[16]. The low-cost and non-toxicity make lactose a practical potential for engineering products. In comparison with IPTG, the parameters of lactose inducing different recombinant protein expression vary greatly and its optimal working conditions are established usually by a large number of laboratory tests[17]. In our previous studies, urease subunit B (UreB) and H pylori adhesin (HpaA) were demonstrated as fine candidates in H pylori engineering vaccine[18,19]. For improving immunogenicity of the vaccine, heat-labile enterotoxin subunit A mutant at the 63rd position (LTKA63) and subunit B (LTB) of Escherichia coli were selected as adjuvants[20]. In this study, 4 constructed prokaryotic expression systems of ureB, hpaA, LTKA63 and LTB were used as the target genes to determine the inducing effects with different lactose dosages, temperatures and times, and original bacterial concentrations on expression of the recombinant proteins.
Four prokaryotic expression systems of pET32a-ureB-E.coliBL21, pET32a-hpaA-E.coli BL21, pET32a-LTKA63-E.coliBL21 and pET32a-LTB-E.coliBL21 were constructed and offered by our laboratory. Tryptone, yeast extract for LB medium were purchased from OXOID (Basingstoke, Hampshire, England). IPTG and lactose used for inducement, and SDS, glycine and DTT were offered by BBST (Shanghai, China). Acrylamide, N, N’-methylen-bis-acrylamid and TEMED were obtained from Serva (Heidelberg, Germany).
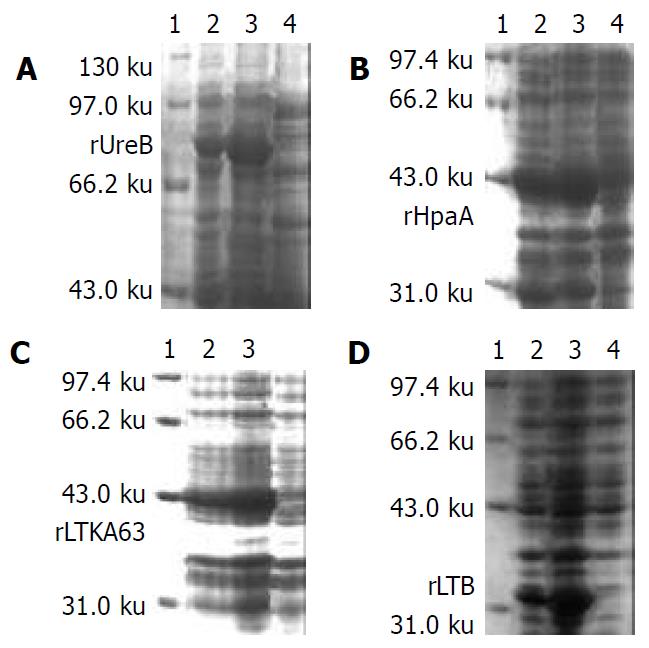
Determination of optimal inducing concentrations of lactose and temperatures A colony of each the four engineering bacteria in LB agar plates was inoculated into 5 mL of LB liquid medium and then incubated on rotator with 200 r/min at 37 °C for 12 h. The values at A600 measured by spectrophotometry were used to indicate the bacterial concentrations. Lactose at the final concentrations of 5, 10, 50 and 100 g/L were added into the cultures of the 4 strains with the A600 values of 1.2, respectively, and then incubated on 200 r/min shaking at 28 °C or 37 °C for 4 h. The bacteria in the medium were collected by centrifugation. By using SDS-PAGE, the expression and outputs of the target recombinant proteins (rUreB, rHpaA, rLTKA63 and rLTB) were examined and estimated, respectively. BIO-RAD gel image analysis system was applied to measure the outputs of target protein fragments by their area percentages in the total bacterial proteins. 0.5 mmol/L IPTG was simultaneously used as an inducer control, which inducing effects for the four recombinant proteins had been confirmed in our previous studies[18-20].
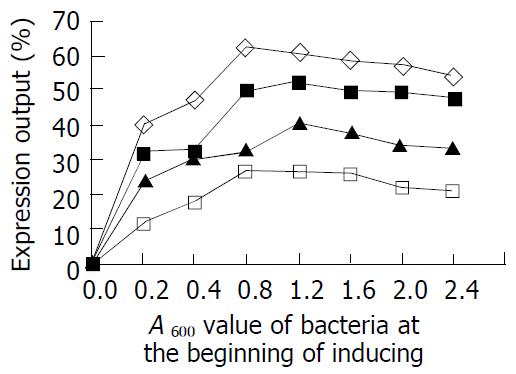
Determination of optimal original bacterium concerntrations for inducement Four engineering bacterial strains were inoculated with proportion of 1:100 (V/V) into LB liquid medium and then incubated on 200 r/min shaking at 37 °C. According to the results obtained above, lactose at the final concentrations of 100, 50, 100 and 100 g/L for inducement of rUreB, rHpaA, rLTKA63 and rLTB expression was added into the cultures with the A600 values of 0.2, 0.4, 0.8, 1.2, 1.6, 2.0 and 2.4, respectively. The lactose added cultures were continuously incubated on 200 r/min shaking at 37 °C for 4 h. The outputs of rUreB, rHpaA, rLTKA63 and rLTB were examined by SDS-PAGE and BIO-RAD gel image analysis system.
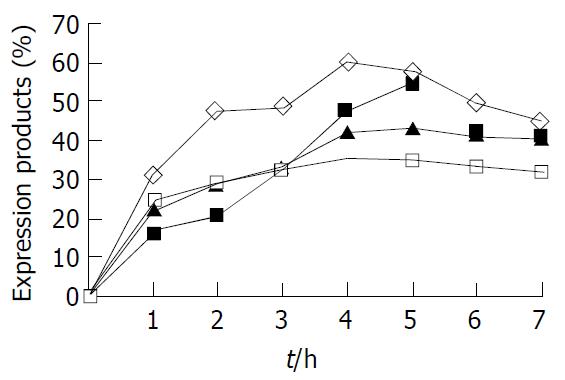
Effects of the target protein expression by using different inducing time According to the results obtained above, the optimal original bacterial concerntration for inducement was 1.2 (A600). The four different bacterial cultures (A600 = 1.2), which expressing rUreB, rHpaA, rLTKA63 or rLTB, were added with lactose at the final concentrations of 100, 50, 100, 100 g/L, respectively. The cultures were continuously incubated on 200 r/min shaker at 37 °C for 1, 2, 3, 4, 5, 6 and 7 h, respectively. The outputs of rUreB, rHpaA, rLTKA63 and rLTB were examined by SDS-PAGE and BIO-RAD gel image analysis system.
SDS-PAGE A vertical discontinious plate polyacrylamide gel eletrophoresis was applied[21]. The isolation gels with 8%, 10%, 12% and 15% concentrations (W/V) were used to detect the expressed rUreB, rLTKA63, rHpaA and rLTB, respectively, based on their different molecular weights. The start voltage was 8 V/cm and then changed to 15 V/cm while the samples went into isolation gels. The gels after electrophoresis were stained by Coomassie brilliant blue and then decolorized with methanol-acetic acid solution.
Lactose with multiple appropriate concentrations could effectively induce expression of 4 target recombinant proteins (Figure 1). Beginning with the original bacterial concentrations of 1.2 A600 values, the outputs of rUreB, rHpaA, rLTKA63 and rLTB at the inducing temperature of 37 °C were 27.3%-53.9%, 7.4%-30.9%, 0.8%-79.8% and 12.7%-119.1% increased as compared with those of 28 °C, respectively (Table 1). When the concentrations of lactose were 100, 50, 100 and 100 g/L inducing at 37 °C for 4 h, outputs of the 4 recombinant proteins reached the highest (Table 1). In comparison with the inducing expression effects of 0.5 mmol/L IPTG, the highest outputs of rUreB, rHpaA, rLTKA63 and rLTB induced by lactose were 203.2%, 98.4%, 114.0% and 245.1% increased, respectively (Table 1).
| Concentrationsof lactose(g/L)and IPTG(mmol/L) | Expression outputs (% of total bacterial proteins) | |||||||
| rUreB | rHpaA | rLTKA63 | rLTB | |||||
| (37 °C ) | (28 °C ) | (37 °C ) | (28 °C ) | (37 °C ) | (28 °C ) | (37 °C ) | (28 °C ) | |
| Lactose (50) | 40.59 | 26.38 | 50.35 | 44.72 | 23.36 | 22.41 | 39.55 | 21.91 |
| (10) | 50.52 | 39.70 | 57.61 | 44.86 | 35.84 | 27.09 | 42.66 | 26.17 |
| (5) | 30.51 | 22.17 | 60.80 | 46.42 | 35.64 | 22.09 | 26.99 | 23.94 |
| (1) | 27.33 | 18.92 | 54.00 | 50.29 | 21.91 | 21.73 | 23.33 | 10.65 |
| (0.5) | 20.78 | 13.63 | 42.29 | 36.05 | 22.01 | 12.24 | 14.72 | 10.31 |
| IPTG (0.5) | 16.66 | 14.72 | 30.65 | 22.61 | 16.75 | 14.48 | 12.36 | 10.06 |
By using the inducing concentrations of lactose with 100, 50, 100 and 100 g/L inducing at 37 °C for 4 h, the expression outputs of the 4 recombinant proteins with the different original bacterial concentrations (0.2-2.4 A600 values) are showed in Figure 2. The results indicated that a better expression effect for any of the 4 recombinant proteins was present when the original bacterial concentration used was higher (A600 = 0.8-1.2).
When the original bacterial concentrations as A600 values of 1.2 and the lactose concentrations of 100, 50, 100 and 100 g/L, the outputs of rUreB, rHpaA, rLTKA63 and rLTB were shown in Figure 3. The results indicated that a higher output for any of the 4 recombinant proteins was present when the inducing time was 4-5 h.
H pylori causes a local superficial infection in human stomach and duodenum[22]. So orally inoculating of H pylori vaccine has a good protective effect[23]. So far, no commercial H pylori engineering vaccine has been available. An engineering vaccine has many advantages but its immunoprotective effect is usually poor because of the unitarity for antigen components. Using adjuvant is an efficient measure to improve immune effect of engineering vaccines[24]. UreB and HpaA were demonstrated to be excellent antigen candidates as their stable and high expression, strong antigenicity, universal distribution in different H pylori isolates and exposure on the surface of the bacterium[25,26]. E. coli LTKA63 and LTB were recently found and were generally considered as the most efficient adjuvants for mucosal immunization so far[27,28]. So we planed to use the rUreB and rHpaA as antigens and LTB or LTKA63 as adjuvants to develop an oral-taken double-valence genetic engineering vaccine of H pylori.
Almost of all the recombinant proteins show a very low expression or non-expression without inducement. IPTG is a routinely laboratory used inducer with high efficiency on recombinant protein expression in E. coli but it must be removed by complicated methods from the induced products because of its toxicity. When at the efficient inducing dosages, the cost of IPTG is approximate hundredfold of lactose. Therefore, lactose as an inducer has industrially remarkable advantages. Lactose, differs from IPTG, is unable to enter the bacterial body. It must be helped by a special enzyme called as primase to be transported into host bacterium. The lactose in bacterial cell must be transferred into allolactose by β- galactosidase and the latter is able to start T7 Lac promotor. In the process inducing expression of recombinant protein, lactose is much more complex than IPTG. So lactose, if used it as an efficient inducer, must be clarified its inducing parameters such as dosage, temperature, time and original bacterial concentration.
It was proved by our study that lactose at the multiple tested concentrations could efficiently induce the expression of rUreB, rHpaA, rLTKA63 and rLTB. The effects of lactose on inducing expression of the 4 recombinant proteins were much stronger than that by IPTG, which demonstrated by 98.4%-245.1% increased outputs (Table 1). Furthermore, it was reported that lactose is a carbon source for bacteria to promote growth and increase number of bacteria in culture, which results in the increase of output of the target recombinant protein.
The results of this study indicated that at 37 °C for 4 h inducement the lactose concentration to obtain the highest expression outputs of rUreB, rHpaA, rLTKA63 and rLTB were 100, 50, 100 and 100 g/L, respectively. It was found in the study that inducing temperatures can obviously affect the expression of the recombinant proteins. For example, with the original bacterial concentration of 1.2 A600 value for 4 h inducement, using 37 °C as the inducing temperature could increase the expression outputs of rUreB, rHpaA, rLTKA63 and rLTB with 27.3%-53.9%, 7.4%-30.9%, 0.8%-79.8% and 12.7%-119.1% of those induced by 28 °C. The original bacterial concentrations when lactose addition was found to affect the outputs of the recombinant proteins. There were preferable expression effects of the recombinant proteins to add lactose when the original bacterial concentrations with the A600 values of 0.8-1.2. With the original bacterial concentration of 1.2 A600 values and at temperature 37 °C, the outputs of the recombinant proteins for 4-5 h inducement by the optimal concentrations of lactose were relatively higher.
Summarily, using lactose as an inducer on the expression of pET32a-ureB-E.coliBL21, pET32a-hpaA-E.coli BL21, pET32a-LTKA63-E.coliBL21 and pET32a-LTB-E.coliBL21, the optimal temperature was 37 °C. The rest optimal parameters for the original bacterial concentrations, dosages of lactose and inducing time were 0.8, 50 g/L and 4 h for rHpaA; 0.8, 100 g/L and 4 h for rLTKA63; 1.2, 100 g/L and 5 h for both rUreB and rLTB, respectively. The results from this study established a beneficial foundation for industrial production of H pylori genetic engineering vaccines.
Edited by Zhang JZ Proofread by Chen WW and Xu FM
| 1. | Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281-284. [PubMed] |
| 2. | Peng ZS, Liang ZC, Liu MC, Ouyang NT. Studies on gastric epithelial cell proliferation and apoptosis in Hp associated gas-tric ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:218-219. |
| 3. | Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Yao YL, Zhang WD. Relation between Helicobacter pylori and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:1045-1049. |
| 5. | Lu SY, Pan XZ, Peng XW, Shi ZL. Effect of Hp infection on gastric epithelial cell kinetics in stomach diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:760-762. |
| 6. | Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its pre-cancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649-651. |
| 7. | Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5-Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669-673. |
| 8. | Cai L, Yu SZ. A molecular epidemiologic study on gastric cancer in Changle, Fujian Province. Shijie Huaren Xiaohua Zazhi. 1999;7:652-655. |
| 9. | Suganuma M, Kurusu M, Okabe S, Sueoka N, Yoshida M, Wakatsuki Y, Fujiki H. Helicobacter pylori membrane protein 1: a new carcinogenic factor of Helicobacter pylori. Cancer Res. 2001;61:6356-6359. [PubMed] |
| 10. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3176] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 11. | Hiyama T, Haruma K, Kitadai Y, Miyamoto M, Tanaka S, Yoshihara M, Sumii K, Shimamoto F, Kajiyama G. B-cell monoclonality in Helicobacter pylori-associated chronic atrophic gastritis. Virchows Arch. 2001;438:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041-2048. [PubMed] |
| 14. | Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy--the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Donovan RS, Robinson CW, Glick BR. Review: optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J Ind Microbiol. 1996;16:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 219] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Yildirim N, Mackey MC. Feedback regulation in the lactose operon: a mathematical modeling study and comparison with experimental data. Biophys J. 2003;84:2841-2851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Menzella HG, Ceccarelli EA, Gramajo HC. Novel escherichia coli strain allows efficient recombinant protein production using lactose as inducer. Biotechnol Bioeng. 2003;82:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Mao YF, Yan J, Li LW. [Cloning, expression and identification of hpaA gene from a clinical isolate of Helicobacter pylori]. Zhejiang Daxue Xuebao Yixueban. 2003;32:9-12. [PubMed] |
| 19. | Chen Z, Yan J, Mao YF. [Construction of prokaryotic expression system of ureB gene from a clinical isolate of Helicobacter pylori and identification of immunogenicity of the fusion protein]. Zhejiang Daxue Xuebao Yixueban. 2003;32:4-8. [PubMed] |
| 20. | Xia XP, Yan J, Zhao SF. [Cloning, expression and identification of Escherichia coli LTB gene and Vibrio cholerae CTB gene]. Zhejiang Daxue Xuebao Yixueban. 2003;32:17-20. [PubMed] |
| 21. | Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. Cold Spring Harbor Laboratory Press. 1989;pp 18.47-18.61. |
| 22. | Recavarren Ascencios R, Recavarren Arce S. [Chronic atrophic gastritis: pathogenic mechanisms due to cellular hypersensitivity]. Rev Gastroenterol Peru. 2002;22:199-205. [PubMed] |
| 23. | Crabtree JE. Eradication of chronic Helicobacter pylori infection by therapeutic vaccination. Gut. 1998;43:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Corthésy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney AC, Haas R, Kraehenbuhl JP, Blum AL, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Opazo P, Müller I, Rollán A, Valenzuela P, Yudelevich A, García-de la Guarda R, Urra S, Venegas A. Serological response to Helicobacter pylori recombinant antigens in Chilean infected patients with duodenal ulcer, non-ulcer dyspepsia and gastric cancer. APMIS. 1999;107:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Verweij WR, de Haan L, Holtrop M, Agsteribbe E, Brands R, van Scharrenburg GJ, Wilschut J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine. 1998;16:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Baudner BC, Giuliani MM, Verhoef JC, Rappuoli R, Junginger HE, Giudice GD. The concomitant use of the LTK63 mucosal adjuvant and of chitosan-based delivery system enhances the immunogenicity and efficacy of intranasally administered vaccines. Vaccine. 2003;21:3837-3844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |