Copyright
©The Author(s) 2022.
World J Gastroenterol. May 7, 2022; 28(17): 1725-1750
Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1725
Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1725
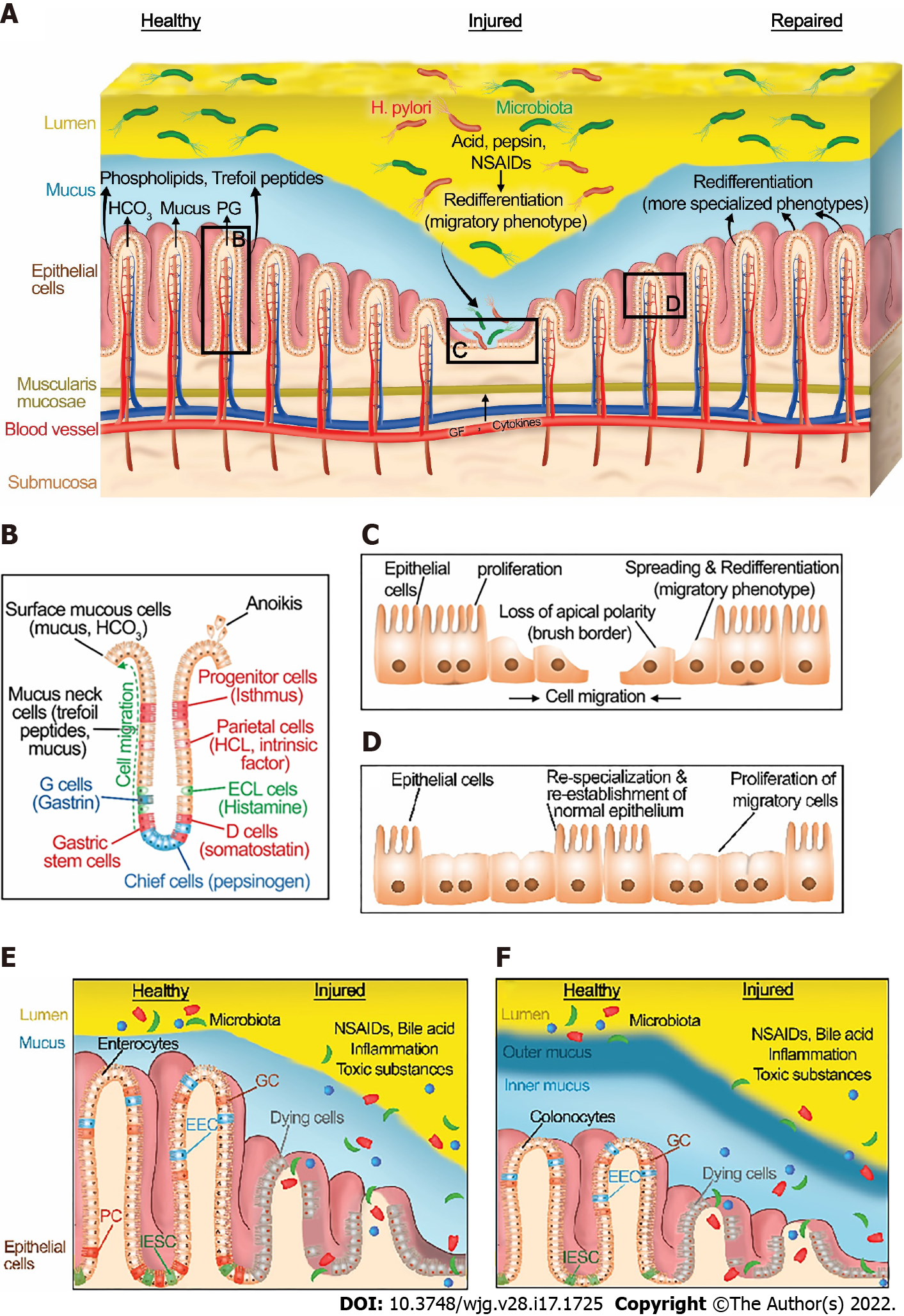
Figure 1 Normal gastrointestinal homeostasis, injury, and healing.
A: Structure of gastric epithelium in healthy, injured, and repaired states. A healthy gastric barrier is essential to maintain gastric homeostasis. In a healthy state, there is an equilibrium between gastric injury and mucosal healing. An excess of destructive factors such as acid, pepsin, nonsteroidal anti-inflammatory drugs (NSAIDs), and H. pylori leads to gastric barrier disruption. These noxious agents then diffuse deeper into the mucosa and create wounds. Epithelial cells at the edge of the injury redifferentiate to a migratory phenotype and collectively migrate as a sheet to close the wound. After successful restitution, the migrated cells redifferentiate to more specialized phenotypes. B: A diagram depicting the structure and cell types of gastric epithelium. C: In the injured state, epithelial cells at the edge of the wound spread and redifferentiate to a migratory phenotype, losing their classical apical brush border and assuming a more squamous morphology. Then, they migrate as a sheet to cover the injured area, with cells at the front of the migrating sheet transmitting traction forces to cells farther back via cell-cell contacts. Epithelial cells behind these migrating cells subsequently proliferate to provide more cells to fully cover larger wounds. D: Cells that have migrated across the defect may themselves then proliferate once the barrier has been reformed. In addition, following migration and proliferation, the migrated cells redifferentiate back to more specialized phenotypes. E: Structure of small intestinal epithelium in healthy and injured states. F: Structure of large intestinal epithelium in healthy and injured states. A healthy intestinal barrier is essential to maintain intestinal homeostasis. In the healthy state, there is an equilibrium between intestinal injury and mucosal healing. An excess of destructive factors such as NSAIDs, inflammation, bile acid, and toxic luminal substances leads to intestinal barrier disruption. These noxious agents then diffuse deeper into the mucosa and create wounds. Epithelial cells at the edge of the injury follow the processes described in the figure legends for in Figure 1C and D. IESC: Intestinal epithelial stem cells; EEC: Enteroendocrine cells; GC: Goblet cells; NSAIDs: Nonsteroidal anti-inflammatory drugs; H. pylori: Helicobacter pylori; PG: Prostaglandins; ECL cells: Enterochromaffin-like cells; PC: Paneth cells; IESC: Intestinal epithelial stem cells.
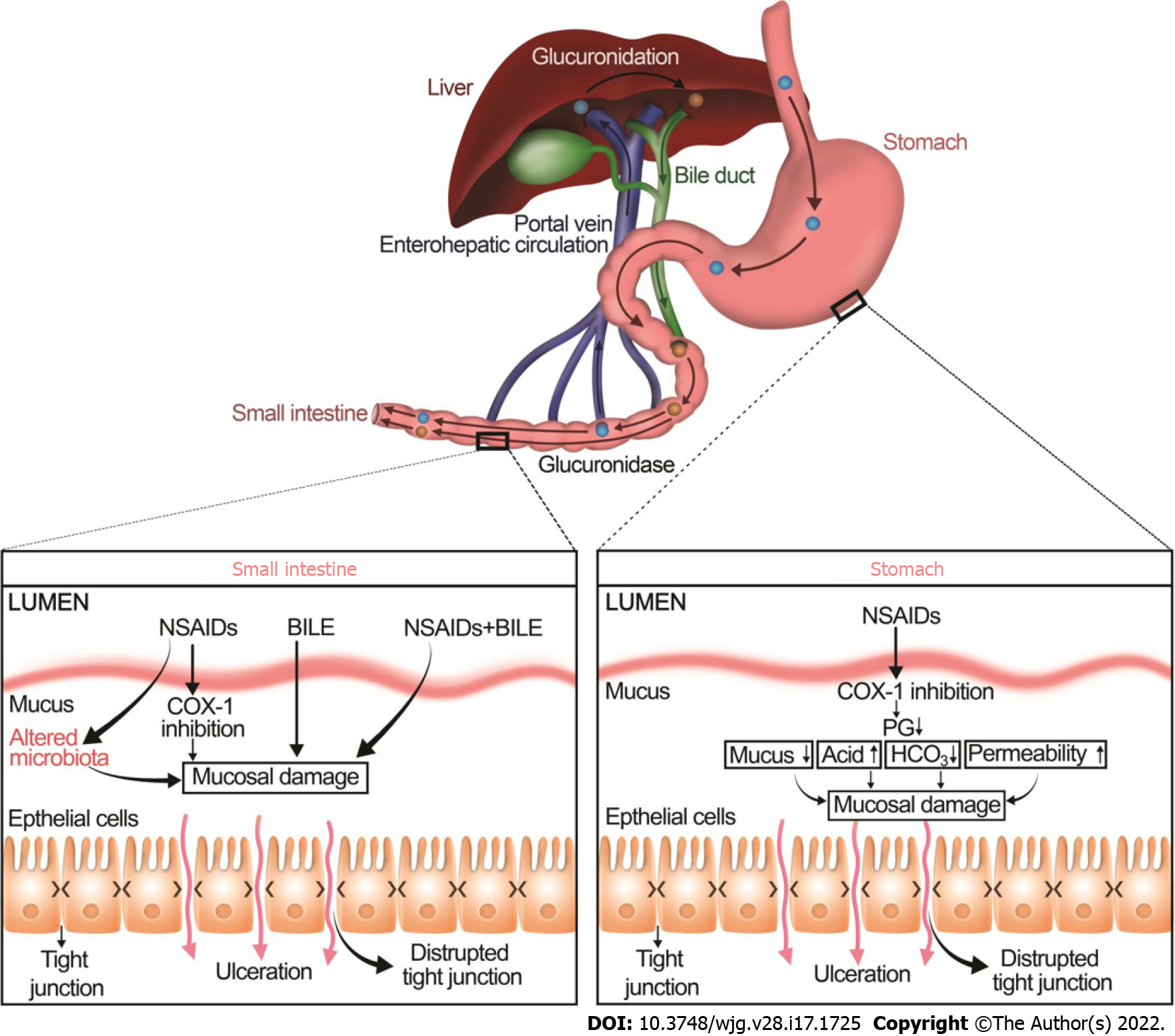
Figure 2 Non-steroidal anti-inflammatory drugs induce mucosal injury in the upper and lower gastrointestinal tract by two distinct mechanisms.
In addition to principal luminal aggressors such as acid, pepsin, Helicobacter pylori in the stomach and acid, bile, and pathogens in the small intestine, nonsteroidal anti-inflammatory drugs (NSAIDs) increase mucosal damage in both upper and lower GI by two different mechanisms. In the stomach, the inhibition of COX-1 by NSAIDs reduces prostaglandin secretion which in turn reduces mucus and bicarbonate secretion and increases acid secretion, resulting in increased permeability and eventually mucosal damage. In the small intestine, NSAIDs bind to bile in the enterohepatic circulation. This potentiates the mucosal damage caused by bile. NSAIDs also increase mucosal damage in the small intestine by altering the gut microbiota. The NSAID-associated increase in enteric gram-negative bacteria appears to contribute to intestinal lesions by increasing inflammation. NSAIDs: Nonsteroidal anti-inflammatory drugs.
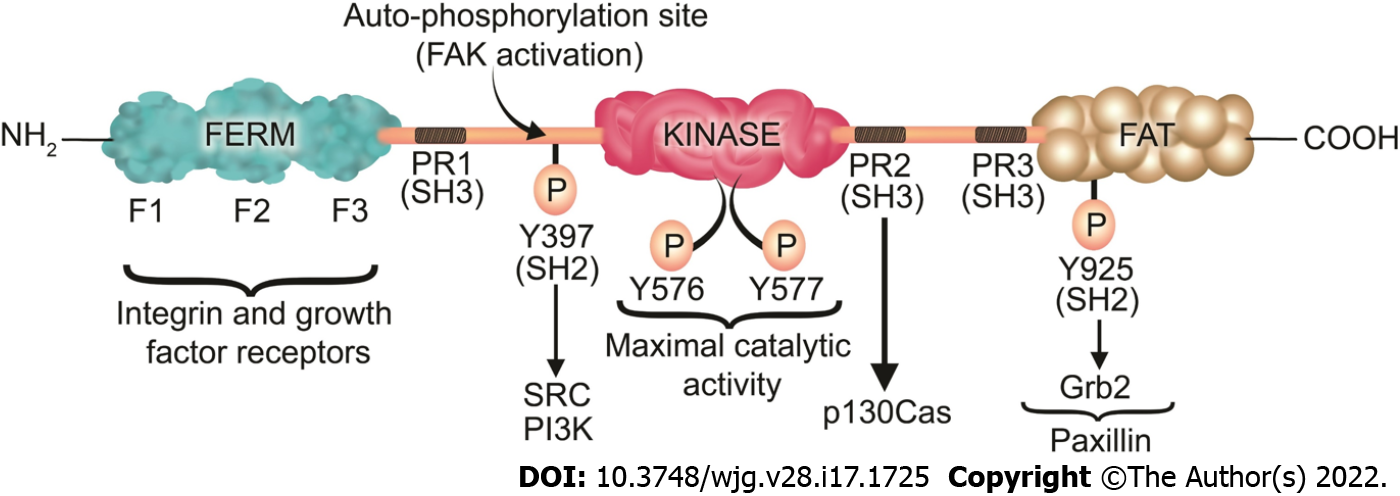
Figure 3 Focal adhesion kinase structure, phosphorylation sites, and its associated proteins.
Focal adhesion kinase (FAK) contains an N-terminal band 4.1-ezrin-radixin-moesin (FERM) domain comprised of three lobes (F1, F2, and F3), a central kinase domain, a C-terminal FAT domain, and two linker domains with three PR regions that bind SH3 domain containing protein such as p130Cas. Y397 is the site of the FAK autophosphorylation, crucial for FAK activation, which interacts with proteins containing the SH2 domain such as Src and PI3K. Subsequently to the SH2 binding, Src binds to the PR1 SH3 domain (PXXP) and further phosphorylates the Y576/577 sites on FAK, which are crucial for the maximal catalytic activity of FAK. Further FAK phosphorylation at Y925 creates a binding site for Grb2. The phosphorylation of FAK-Y-925 and subsequent Grb2 binding disassociates paxillin from FAK, which results in FAK release from FAs, thus stimulating FA disassembly. The FERM domain regulates the interactions of FAK with growth factor receptors and integrins. The FAT domain recruits FAK to FAs by associating with paxillin. FERM: Band 4.1-ezrin-radixin-moesin; FAT: Focal adhesion targeting; PR: Proline-rich region; SH: Src homology; P: Phosphorylation.
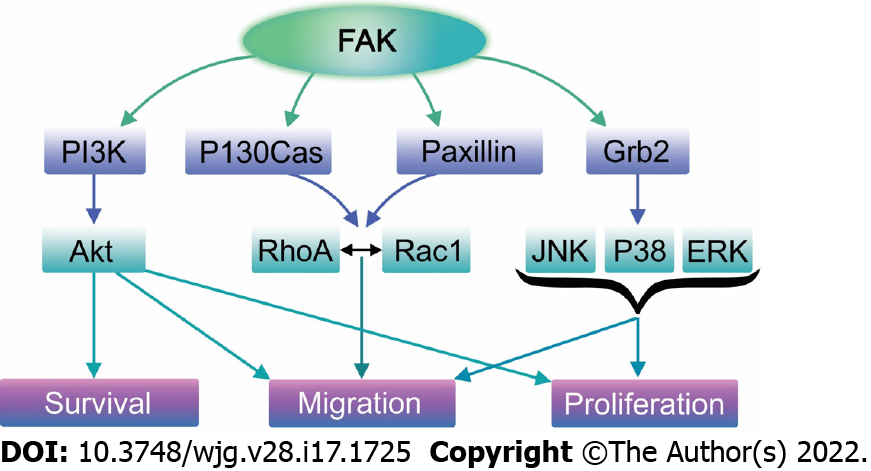
Figure 4 Focal adhesion kinase plays a crucial role in several signaling pathways that promote migration, proliferation, and survival.
Upon its activation, focal adhesion kinase (FAK) directly binds PI3K, leading to the activation of Akt. Activated Akt then stimulates numerous cellular functions including cell survival, proliferation, and migration via various signaling cascades depending on the cell type and species. In addition, FAK may recruit Grb2 and subsequently activate the Ras/Raf/MAPK pathway, enhancing cell proliferation and motility. Finally, FAK may directly bind to paxillin and p130Cas, promoting lamellipodium formation, and thus migration via Rac GTPase activation.
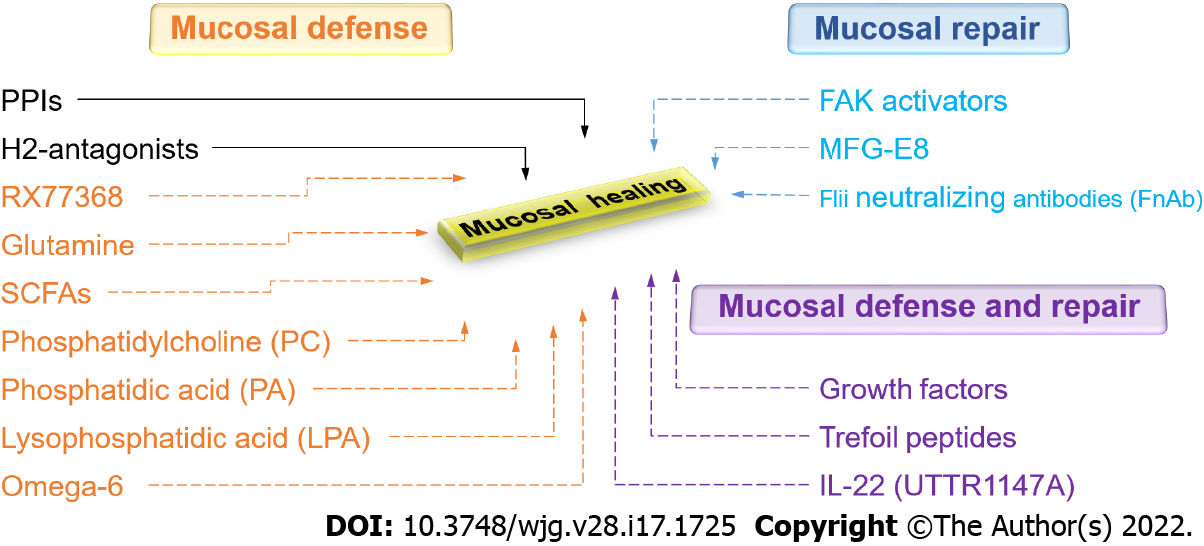
Figure 5 Current and promising new therapeutic approaches to gastrointestinal mucosal healing.
Green represents currently available drugs. Red represents promising new therapeutic approaches that increase mucosal defense. Blue represents promising new therapeutic approaches that promote mucosal repair. Purple represents promising new therapeutic approaches that stimulate both mucosal defense and repair. PPIs: Proton pump inhibitors; H2-antagonists: Histamine-2 receptor antagonists; RX77368: The thyrotropin-releasing hormone analog; SCFAs: Short-chain fatty acids; FAK: Focal adhesion kinase; MFG-E8: Milk fat globule-epidermal growth factor 8; Flii: Flightless I.
- Citation: Oncel S, Basson MD. Gut homeostasis, injury, and healing: New therapeutic targets. World J Gastroenterol 2022; 28(17): 1725-1750
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1725