Copyright
©The Author(s) 2021.
World J Gastroenterol. Oct 28, 2021; 27(40): 6927-6938
Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6927
Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6927
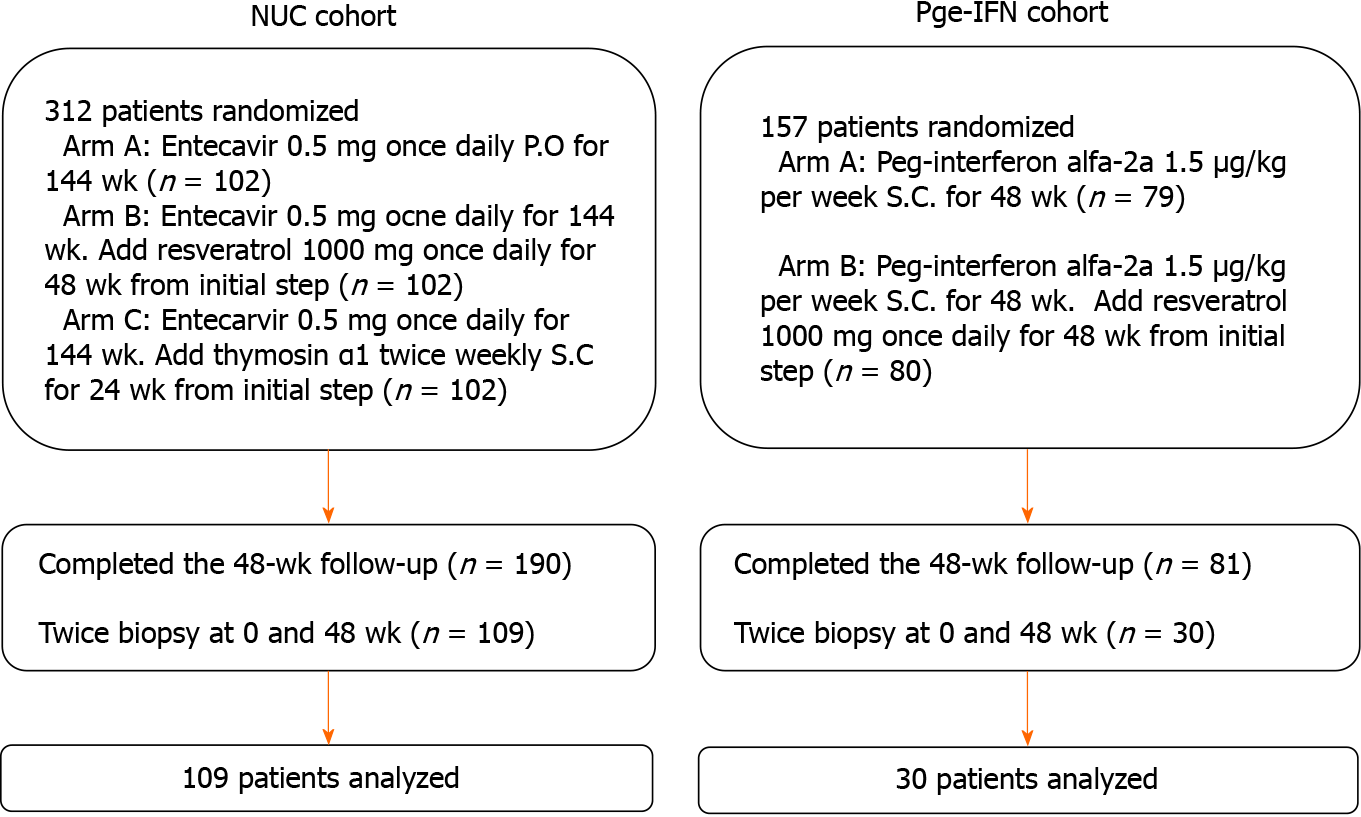
Figure 1 Patient flowchart.
NUC: Nucleos(t)ide analog; Peg-IFN: Peginterferon.
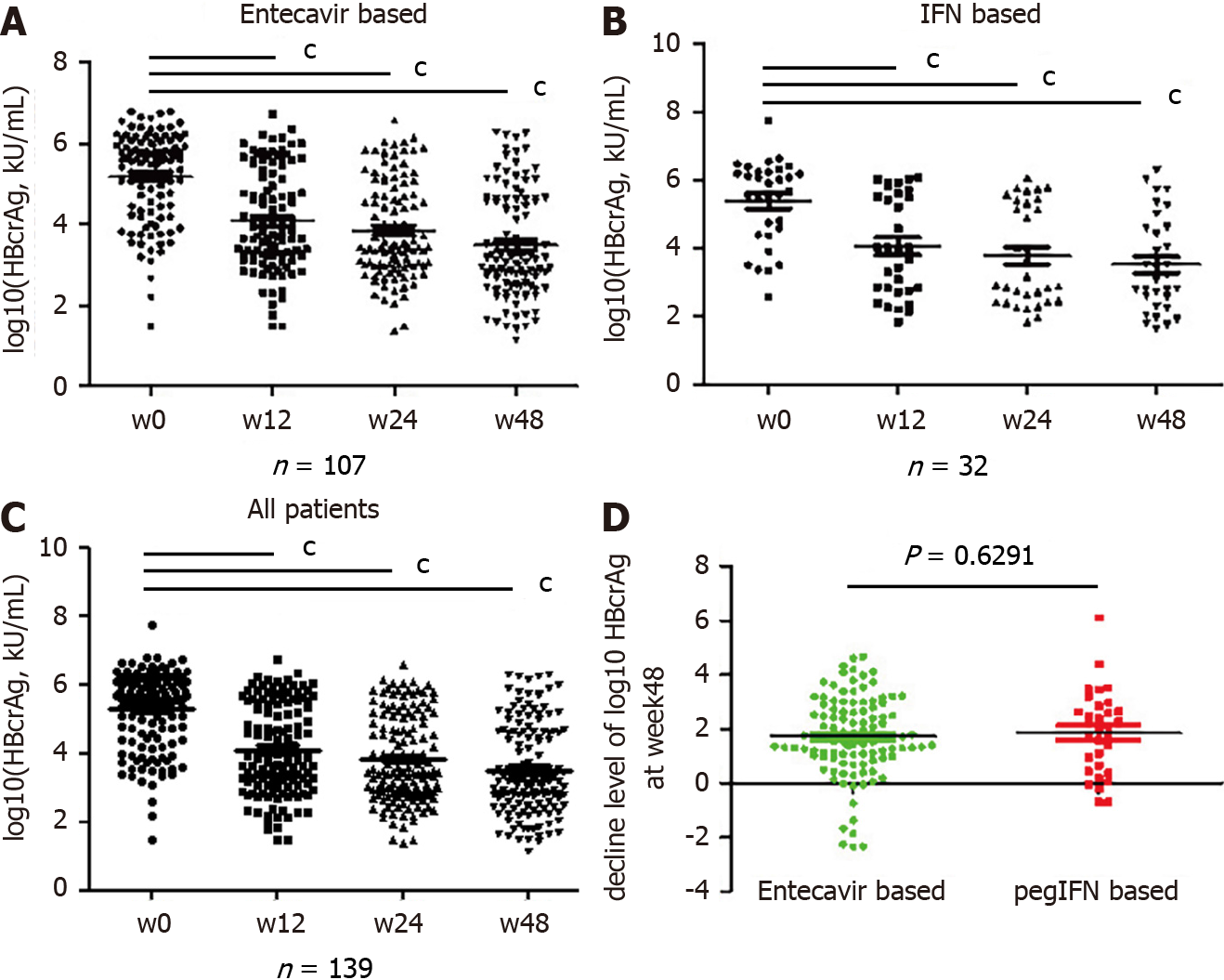
Figure 2 Time-dependent distribution of serum quantitative hepatitis B core-related antigen in patients with chronic hepatitis B and treated with different regimens.
A: Changes in hepatitis B core-related antigen (HBcrAg) levels during treatment in the entecavir group; B: Changes in HBcrAg levels during treatment in the peginterferon group; C: Changes in HBcrAg levels during treatment in all patients; D: Comparison of the decrease in HBcrAg levels from baseline to week 48 between the entecavir and peginterferon groups. cP < 0.0001. HBcrAg: Hepatitis B core-related antigen; IFN: Interferon.
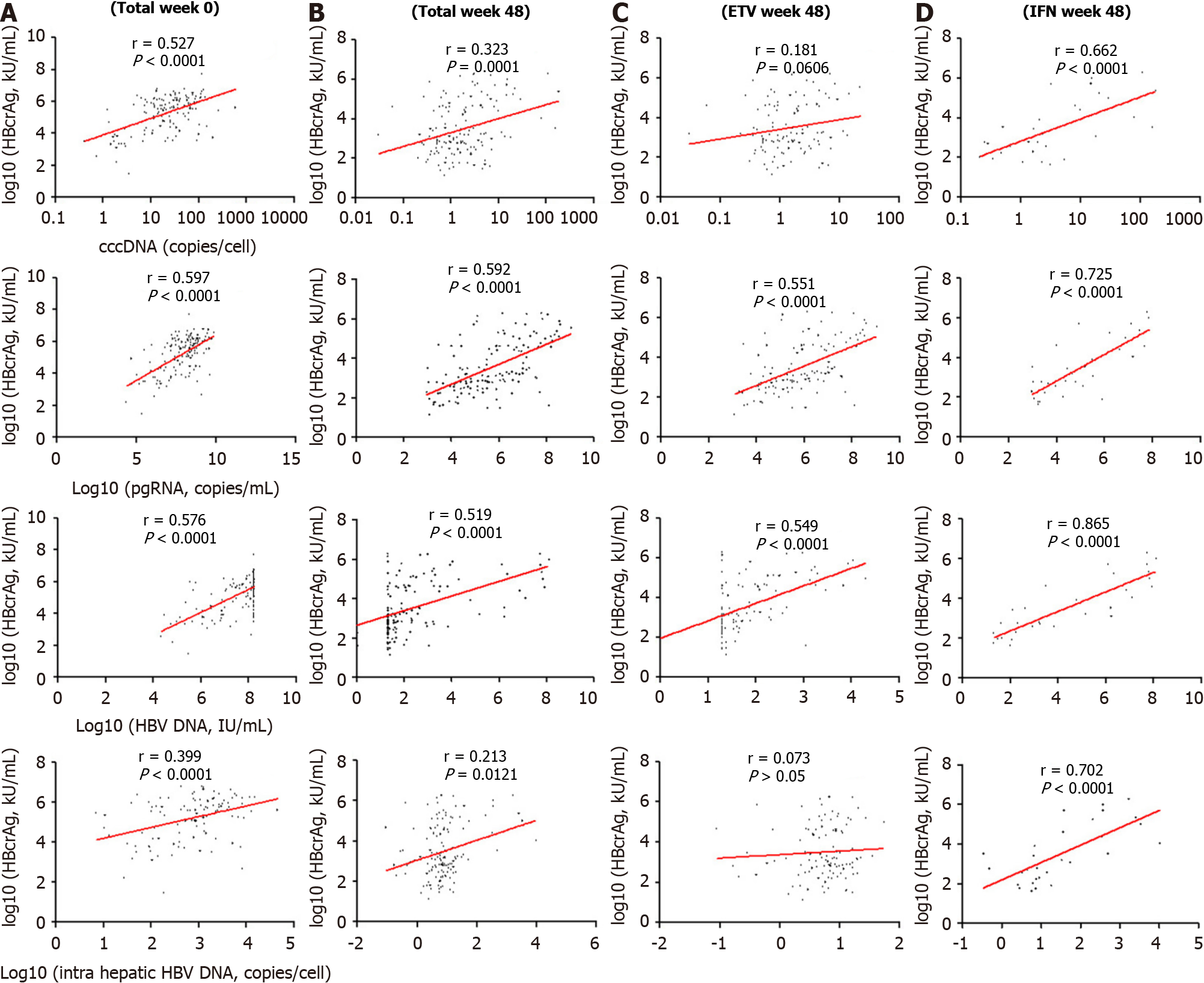
Figure 3 Correlation of serum quantitative hepatitis B core-related antigen with covalently closed circular DNA, pregenomic RNA, serum, and intrahepatic hepatitis B virus DNA level.
A: In all patients at baseline; B: In all patients at week 48; C: Entecavir at week 48; D: Peginterferon at week 48. cccDNA: Covalently closed circular DNA; ETV: Entecavir; HBV: Hepatitis B virus; IFN: Interferon. pgRNA: Pregenomic RNA.
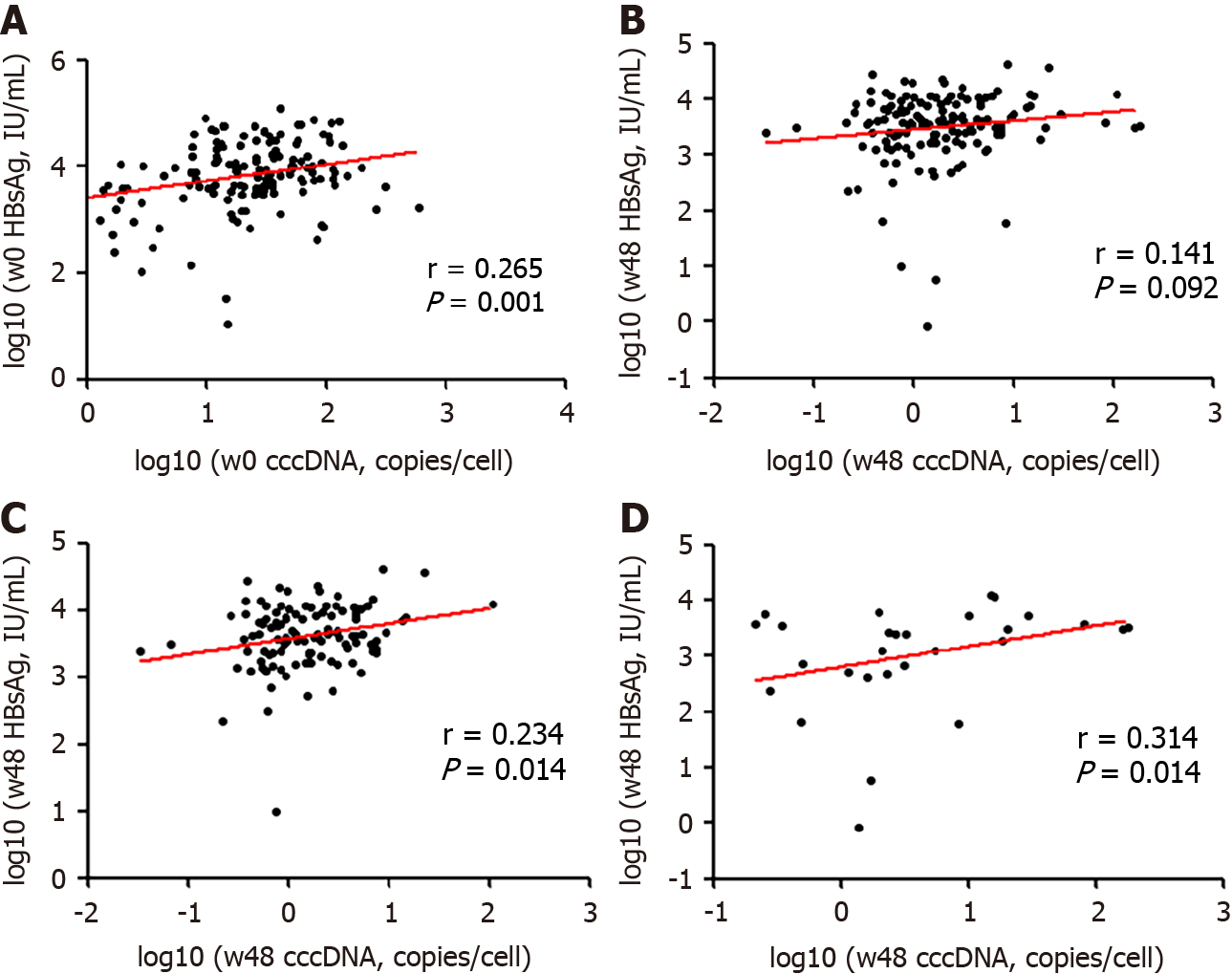
Figure 4 Correlation of serum hepatitis B surface antigen with covalently closed circular DNA.
A: Correlation between serum hepatitis B surface antigen (HBsAg) and intrahepatic covalently closed circular DNA (cccDNA) at baseline; B: Correlation between serum HBsAg and intrahepatic cccDNA and at week 48; C: Correlation between cccDNA and HBsAg levels at weeks 48 in the entecavir cohort; D: Correlation between cccDNA and HBsAg levels at weeks 48 in the interferon cohort. HBsAg: Hepatitis B surface antigen; cccDNA: Covalently closed circular DNA.
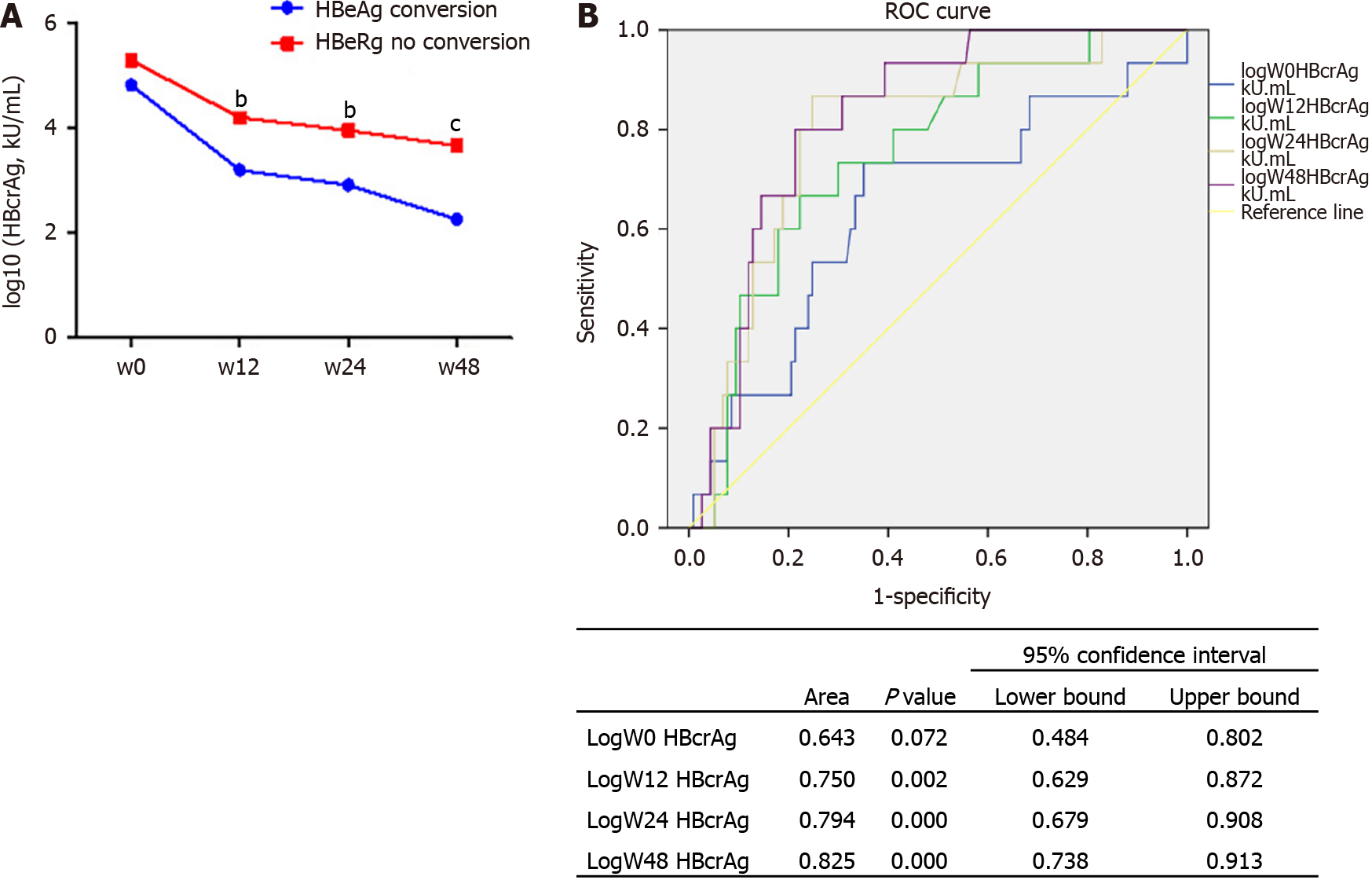
Figure 5 Hepatitis B core-related antigen prediction of hepatitis B e antigen seroconversion at 48 wk.
A: Concentration of hepatitis B core-related antigen (HBcrAg) at different times in the two groups of hepatitis B e antigen (HBeAg) seroconversion; B: Receiver operating characteristic curve analysis of HBcrAg to predict HBeAg seroconversion. HBcrAg: Hepatitis B core-related antigen; HBeAg: Hepatitis B e antigen; ROC: Receiver operating characteristic; W: Week.
- Citation: Chi XM, Wang XM, Wang ZF, Wu RH, Gao XZ, Xu HQ, Ding YH, Niu JQ. Serum hepatitis B core-related antigen as a surrogate marker of hepatitis B e antigen seroconversion in chronic hepatitis B. World J Gastroenterol 2021; 27(40): 6927-6938
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6927.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6927