Copyright
©The Author(s) 2021.
World J Gastroenterol. Aug 7, 2021; 27(29): 4763-4783
Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4763
Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4763
Figure 1 Severe acute respiratory syndrome coronavirus 2 infection induces microbial dysbiosis and intestinal inflammation.
Severe acute respiratory syndrome coronavirus 2 infections of intestinal epithelial cells result in a pro-inflammatory immune response leading to infiltration of inflammatory lymphocytes and disrupting the gut barrier. This disruption to homeostasis allows overgrowth of detrimental bacteria resulting in dysbiosis, and the impaired gut barrier facilitates the translocation of bacteria exacerbating the inflammatory response. Additionally, opportunistic fungal infections have been found in some patients, further contributing to the dysfunction. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
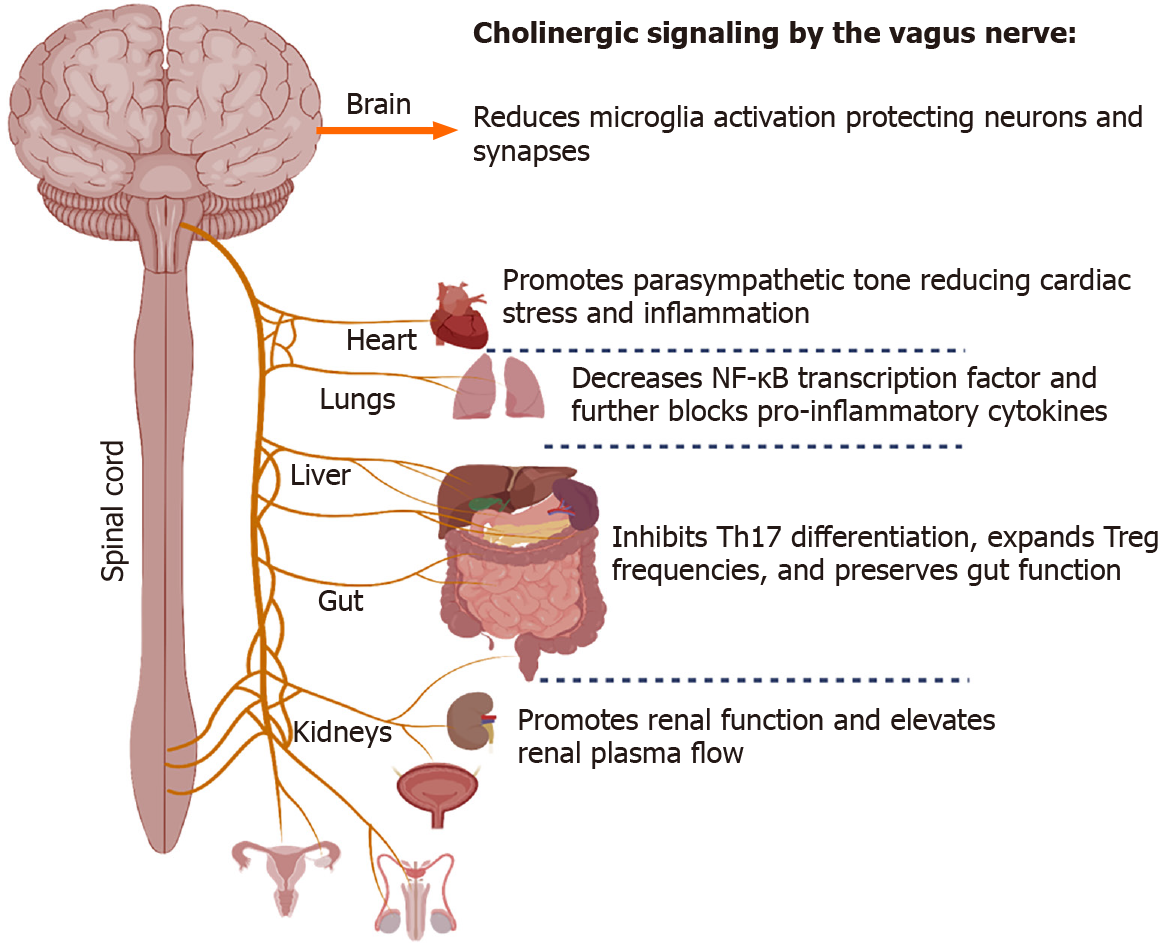
Figure 2 Role of acetylcholine on immune micro-environments in diverse tissues including the brain, heart, lungs, and gut.
Cholinergic signaling can be exploited to reduce microglia activation in the central nervous system, lower systemic inflammation, and promote optimal organ function in diseased states such as severe coronavirus disease 19 infection where systemic dysfunction occurs.
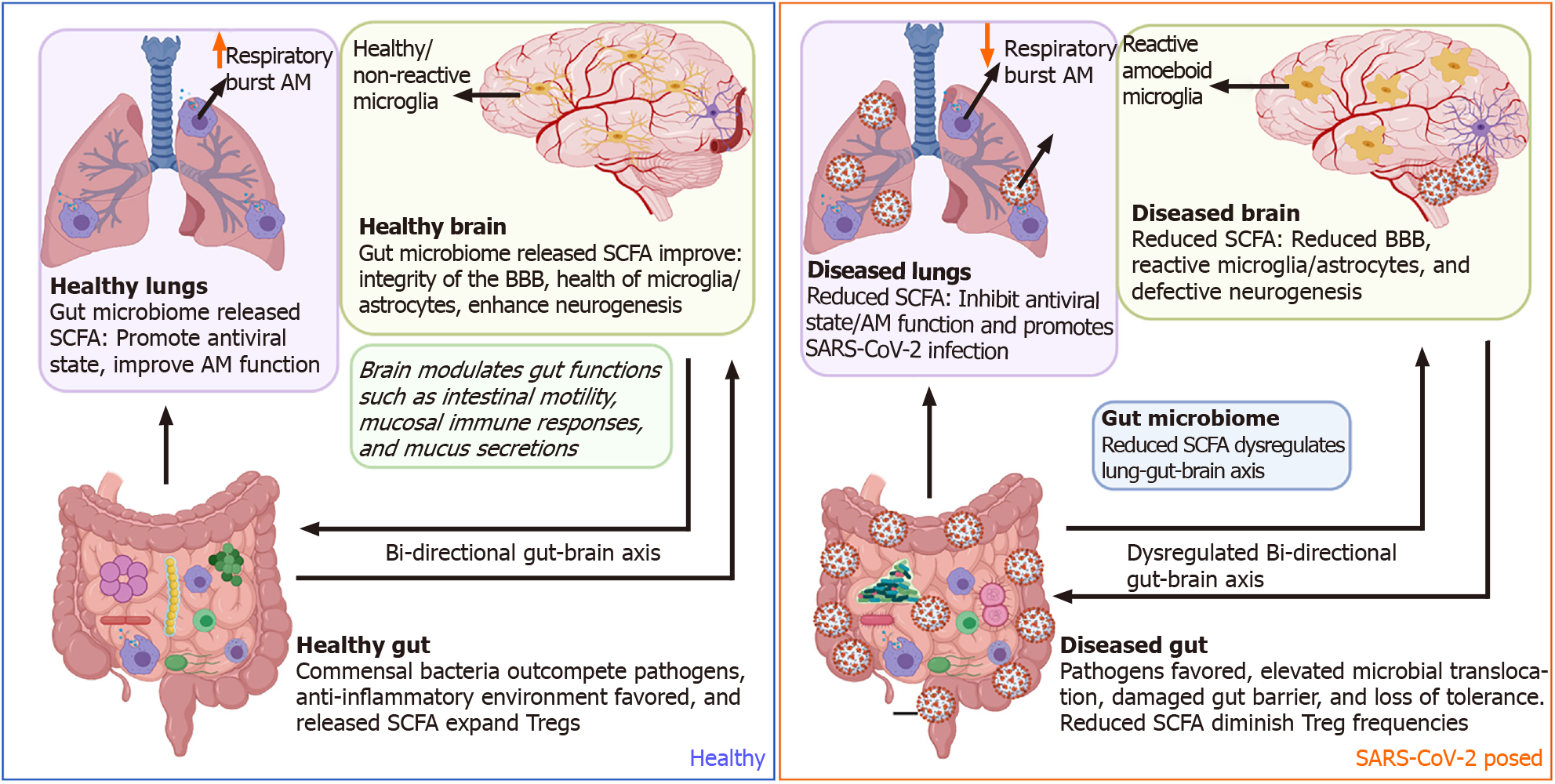
Figure 3 Potential mechanisms by which severe acute respiratory syndrome coronavirus 2 dysregulates the gut-brain axis.
Side by side comparisons of the gut-brain-lung axis in a healthy state vs a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposed state. In a healthy state, commensal bacteria outcompete pathogens within the gut micro-environment leading to a predominantly anti-inflammatory state. Peptides released by commensal gut bacteria support optimal brain and lung function. During SARS-CoV-2 infection, gut microbial dysbiosis dysregulates gut, lung, and brain function. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; BBB: Blood-brain barrier; AM: Alveolar macrophages; SCFA: Short-chain fatty acids; T-regs: T regulatory cells.
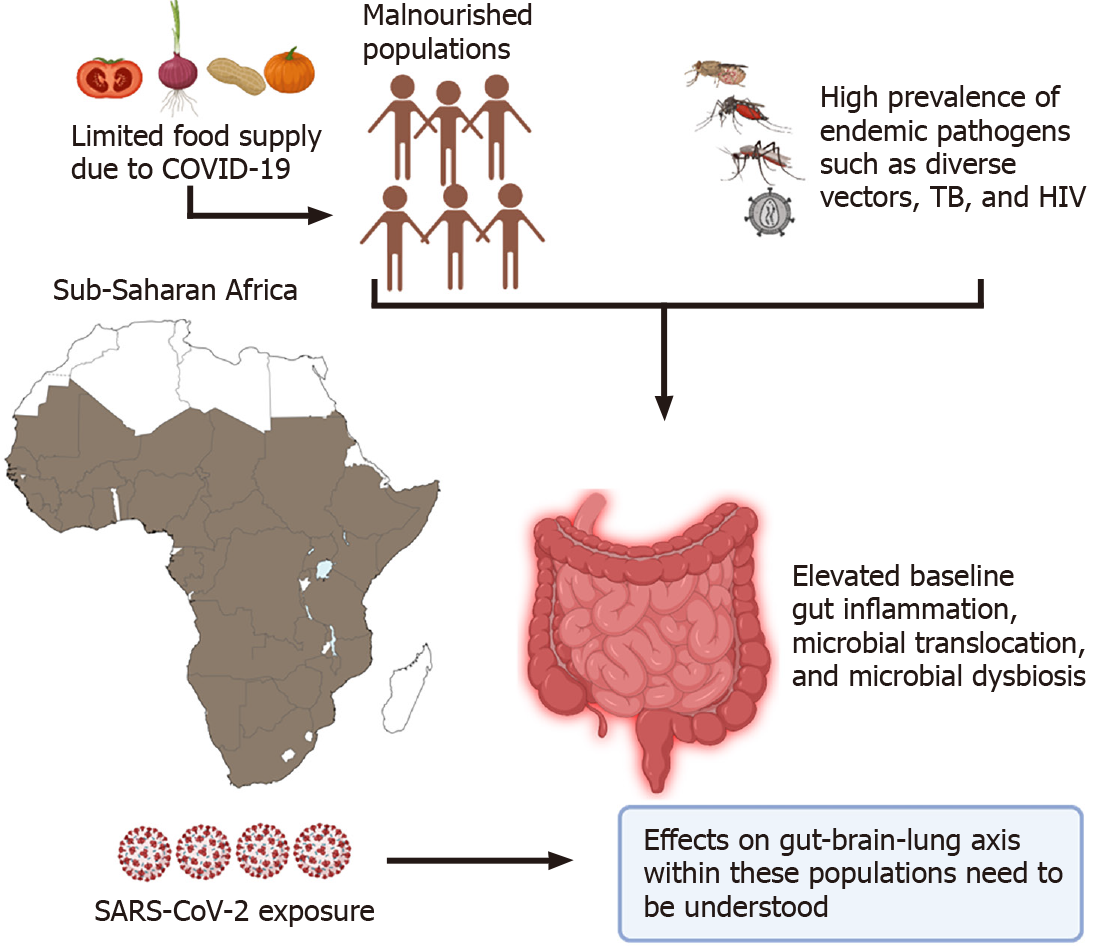
Figure 4 Elevated baseline gut inflammation observed in Sub-Saharan African individuals could lead to severe coronavirus disease 19 associated dysregulation of the gut-brain-lung axis.
Continuous exposure to various endemic pathogens such as human immunodeficiency virus, tuberculosis, multiple vectors like tsetse flies, and several mosquitoes, in addition to low diets, set the ground for high levels of gut dysbiosis within the entire community populations. Additional studies focused on evaluating the effects of baseline gut dysbiosis on coronavirus disease 19 infection are highly warranted. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 19, HIV: Human immunodeficiency virus; TB: Tuberculosis.
- Citation: Johnson SD, Olwenyi OA, Bhyravbhatla N, Thurman M, Pandey K, Klug EA, Johnston M, Dyavar SR, Acharya A, Podany AT, Fletcher CV, Mohan M, Singh K, Byrareddy SN. Therapeutic implications of SARS-CoV-2 dysregulation of the gut-brain-lung axis. World J Gastroenterol 2021; 27(29): 4763-4783
- URL: https://www.wjgnet.com/1007-9327/full/v27/i29/4763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i29.4763