Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Jun 21, 2014; 20(23): 7137-7151
Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7137
Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7137
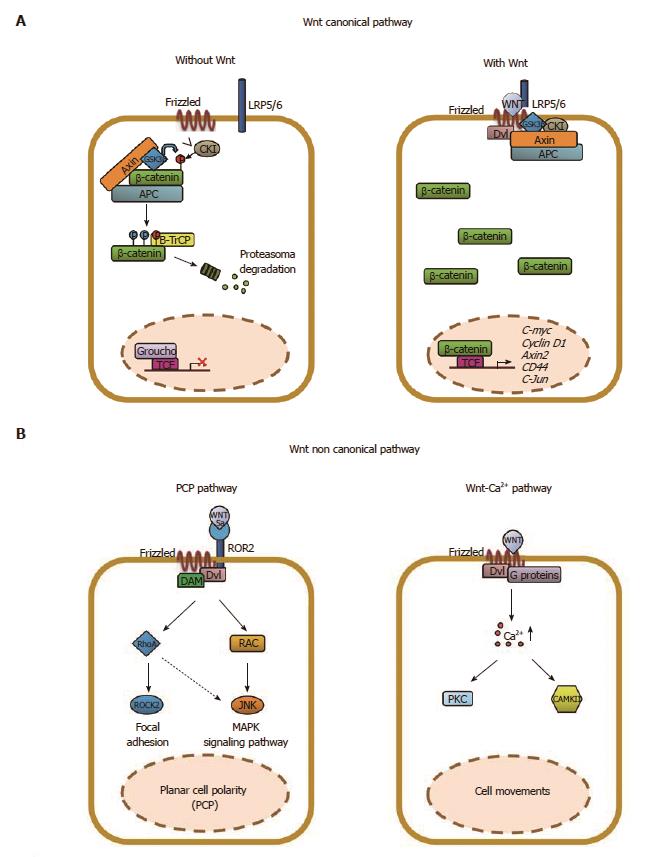
Figure 1 Schematic representation of the Wnt/β-catenin signaling in epithelial cells.
The Wnt signaling pathway can be subdivided into a “canonical” or β-catenin-dependent and “non-canonical” or β-catenin-independent. A: In the absence of Wnt ligands, a multi-subunit destruction complex, composed by adenomatous polyposis coli (APC), Axin, GSK3β, CKI, binds and phosphorylates β-catenin tagging for ubiquitination and subsequent proteasomal degradation (βTrCP). The “canonical” Wnt signaling is initiated by the binding of one of 19 Wnt ligands to one of 10 Frizzled receptors (Fzd), in the presence of the co-receptor LRP5 or 6. This leads to recruitment of Disheveled and inhibition of the APC destruction complex. Accumulation of β-catenin in the cytoplasm leads to its translocation to the nucleus where it interacts with TCF/LEF to drive transcription of Wnt target genes including c-myc, cyclin D1, axin2 and others; B: The “non-canonical” Wnt signaling is initiated by the binding of Wnt5a to ROR2, alone or in combination, with a Frizzled receptor leading to the activation of the planar-cell polarity (PCP) pathway through Rock2, RhoA, Rac or JNK. Alternatively, Wnt11 can bind a Frizzled receptor alone and activate the Wnt/calcium pathway that involves the calcium/calmodulin dependent Kinase II (CamKII), protein-kinase-C (PKC) and nuclear factor of activated T cells (NFAT). Importantly, the “non-canonical” Wnt pathway inhibits the “canonical“ one either impairing β-catenin accumulation in the cytoplasm or the β-catenin/TCF/LEF complex formation.
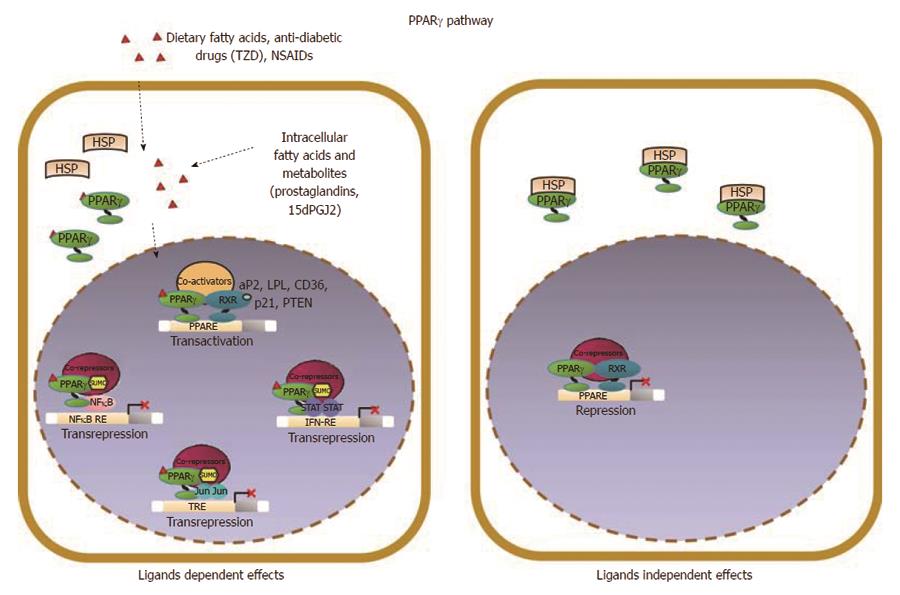
Figure 2 Schematic model of PPARγ signaling in epithelial cells.
PPARγ acts as a pro-differentiating transcription factor in colonic epithelial cells where it is abundantly expressed. A variety of endogenous and exogenous compounds, including lipophilic molecules such as polyunsaturated fatty acids and prostaglandines, have been identified as PPARγ ligands; in particular, 15-deoxy-Δ12,14-PGJ2 (15dPGJ2), is considered a natural ligand for PPARγ. Two molecular mechanisms have been proposed to explain PPARγ effects in maintaining cellular differentiation and homeostasis referred to as PPARγ ligand-dependent or PPARγ ligand-independent effects. (1) in the ligand-dependent transactivation, PPARγ binds the cognate PPRE as heterodimer with RXR and activates target gene expression (PTEN, p21, CDH1) through the recruitment of coactivators; (2) an alternative mode of action is known as ligand-dependent trans-repression, in which the SUMOylated form of the receptor interacts with transcription factors such as NFκB, STAT or JUN and represses their target genes transcription. This is attained through the recruitment and stabilization of corepressor complexes at the promoter regions of proinflammatory or protumorigenic genes by a functionally distinct pool of PPARγ that is specifically SUMOylated at susceptible aminoacid residues in the presence of selected agonists.
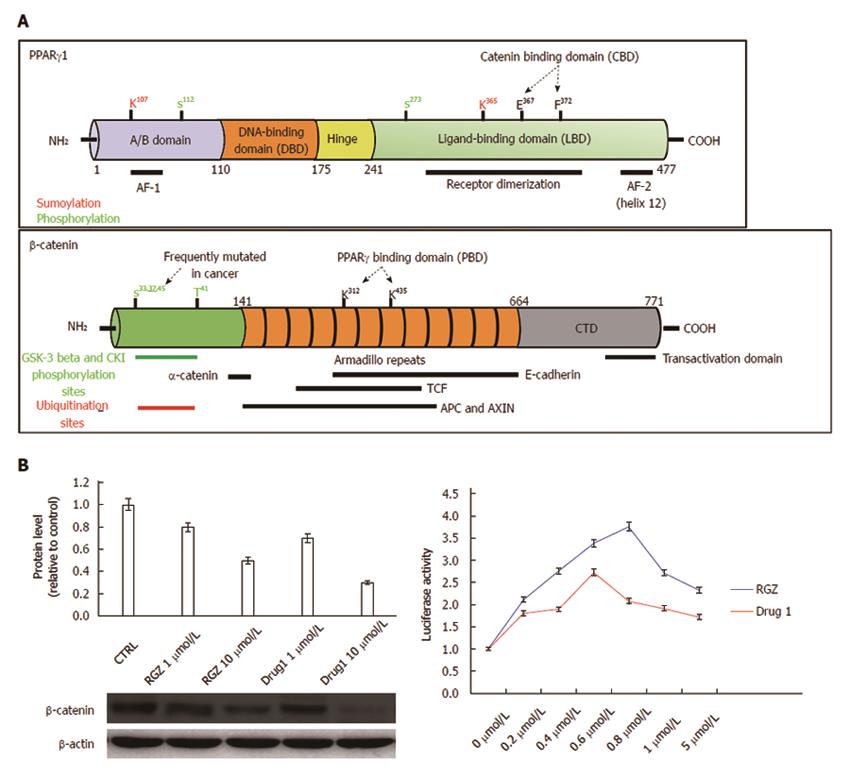
Figure 3 Structural and functional domains of PPARγ and β-catenin.
A: The mature PPARγ protein consists of four structural/functional domains: (1) the variable A/B region at the N terminus contains the ligand-independent transactivation domain AF1 (residues 1–71 of PPARγ1); lysine 79 and serine 84 residues are targets of SUMOylation and phosphorylation events, respectively; (2) the C region is the DNA binding domain, characterized by two C4 Zinc-finger motifs, that interact with the major groove of the DNA; (3) the D or hinge region allows receptor dimerization and DNA binding; and (4) the E/F region is the ligand binding domain (LBD) constituted by 12 α-helices and 4 β-strands where the agonist accommodates. This region (helices 7 and 8) includes a β-catenin binding domain (CBD) essential for the interaction with β-catenin. The most important aminoacid residues implicated in PPARγ activity regulation are shown. The full length β-catenin is essentially composed by three domains: (1) the N-terminal domain involved in the ubiquitin-mediated degradation; (2) the arm repeat domain, containing 12 armadillo repeats that mediate the binding with cadherins, APC, TCF/LEF, CREB binding protein (CBP) and PPARγ; and (3) the carboxy terminal (CTD) or transactivating domain interacts with coactivators such as CBP or corepressors such as β-catenin inhibitor and TCF-4 (ICAT). The most important aminoacid residues implicated in β-catenin activity regulation are shown; B: Luciferase activity from HEK293T cells transfected with a PPRE-driven luciferase reporter gene and exposed to the compound indicated as Drug1 is lower than that obtained from cells exposed to rosiglitazone, indicating a reduced transactivation potential in line with the notion of a partial agonist. HT29 colon cancer cells treated with Drug1 exhibit inhibition of cell growth and a 40% higher ability to downregulate β-catenin than rosiglitazone, likely through a mechanism involving β-catenin nuclear export and proteasome-mediated degradation.
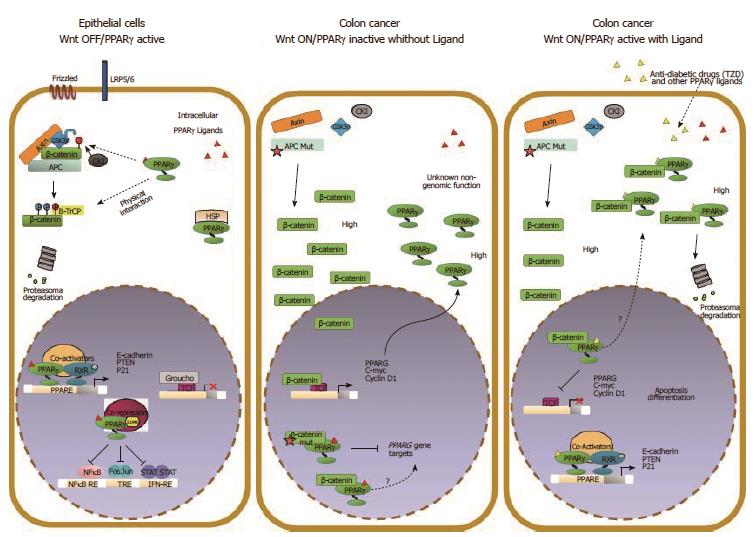
Figure 4 Molecular interactions between Wnt/β-catenin and PPARγ signaling in colorectal cancer cells.
The Wnt/β-catenin and PPARγ signal transduction pathways likely act in a coordinated manner to ensure epithelial cells a balance between growth and differentiation. In this condition, β-catenin is targeted by PPARγ for phosphorylation and subsequent degradation. In CRC, the Wnt pathway is generally overactive and β-catenin is stabilized and translocates to the nucleus to activate Wnt target genes. In a “Wnt on” state, PPARγ protein is generally elevated likely due to high β-catenin levels. The selective inhibition of PPARγ target genes expression may be ascribed to different mechanisms: interaction of β-catenin with PPARγ-associated transcriptional complexes recruited on the DNA that results in transcription inhibition or in squelching of critical PPARγ coactivators through the alternative binding with β-catenin. Our data and those already published suggest a hypothetical model whereby a ligand-bound PPARγ suppresses Wnt/β-catenin signaling in a cancer-cell context dependent manner by: (1) activating transcription of its own target genes; (2) facilitating the GSK3β-dependent degradation of β-catenin; and (3) competing in the nucleus with transcription factors such as LEF/TCFs in blocking prosurvival β-catenin target genes also in cells harboring a mutated APC.
- Citation: Sabatino L, Pancione M, Votino C, Colangelo T, Lupo A, Novellino E, Lavecchia A, Colantuoni V. Emerging role of the β-catenin-PPARγ axis in the pathogenesis of colorectal cancer. World J Gastroenterol 2014; 20(23): 7137-7151
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7137