Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 14, 2012; 18(14): 1579-1589
Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1579
Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1579
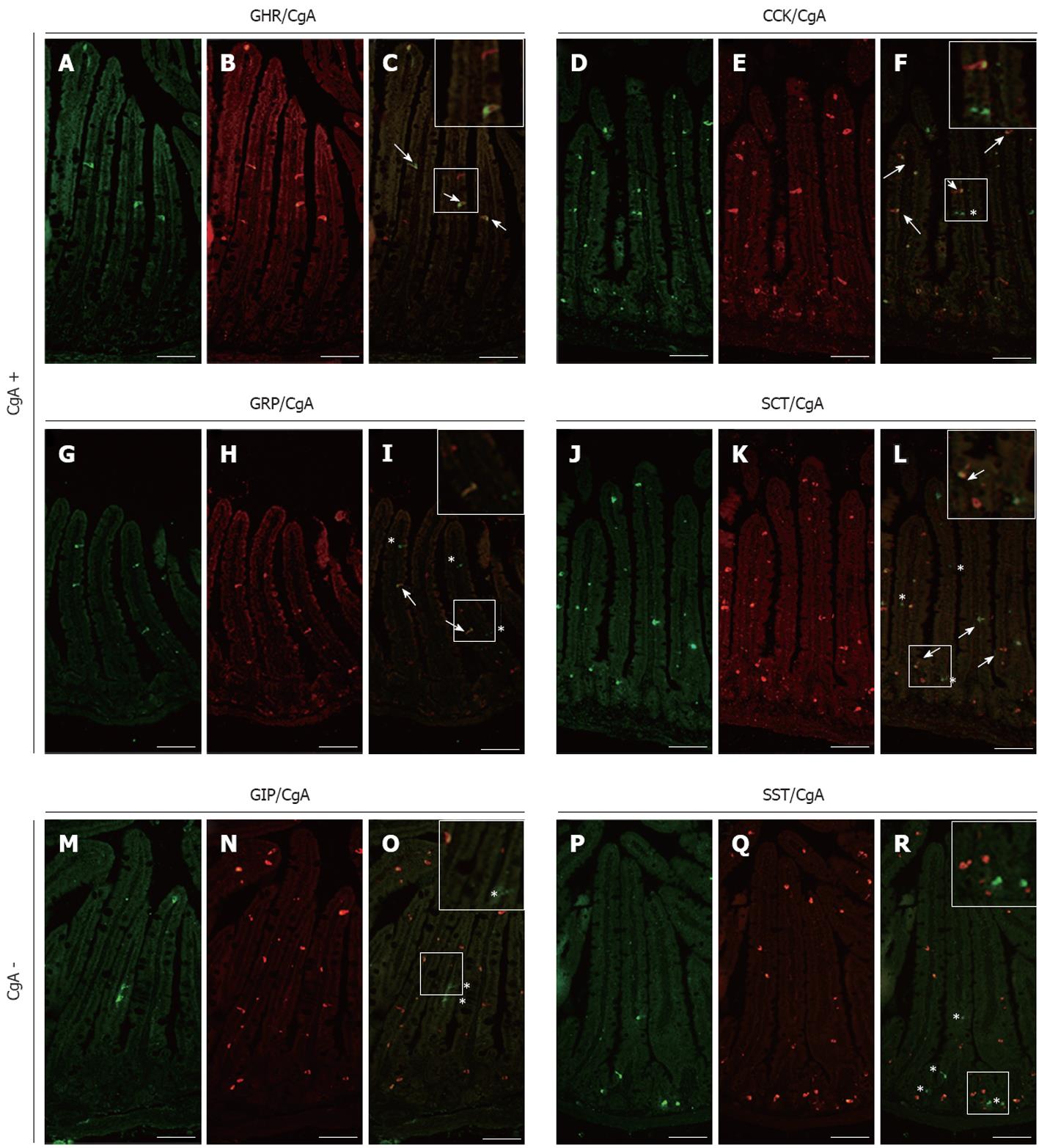
Figure 1 Analysis of chromogranin A co-expression in mouse small intestinal endocrine subpopulations.
Small intestine sections of adult control mice were co-immunostained with antibodies directed against ghrelin (GHR) (A), cholecystokinin (CCK) (D), gastrin-releasing peptide (GRP) (G), secretin (SCT) (J) glucose-dependent insulinotropic peptide (GIP) (M) or somatostatin (SST) (P) and against chromogranin A (CgA) (respectively B, E, H, K, N and Q). The arrows in images C, F, I and L show co-expression of CgA respectively with GHR, CCK, GRP and SCT while asterisks in images F, I, L, O and R point to CgA-negative enteroendocrine cells. The number of arrows and asterisks within the crypt-villus axis represents the average proportion of labelled cells per units. Scale bar: 50 μm.
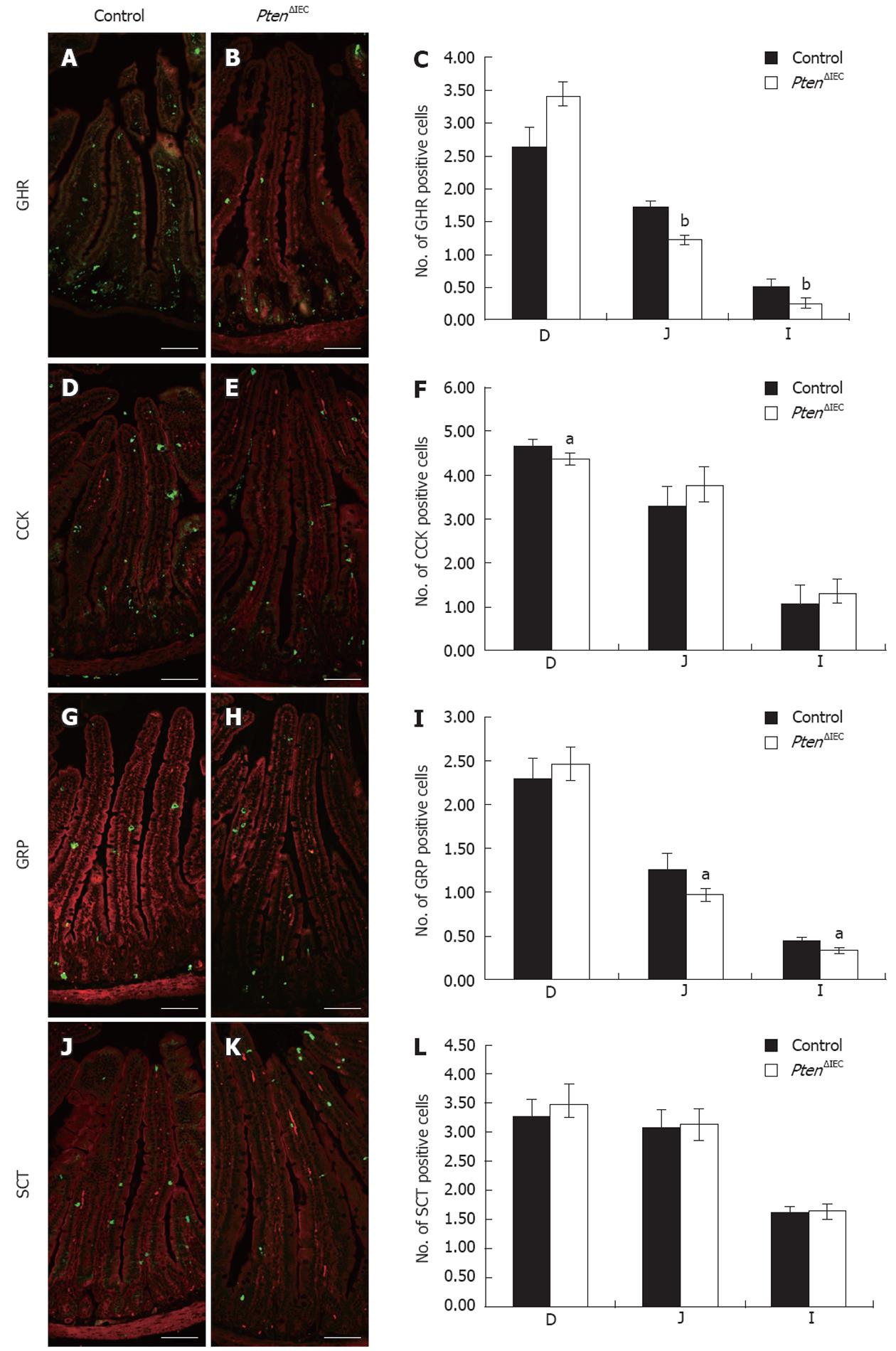
Figure 2 Epithelial Pten positively regulates commitment of chromogranin A-positive enteroendocrine subpopulations in the small intestine.
Duodenum, jejunum and ileum of adult control and Pten∆IEC mice were immunostained with antibodies against ghrelin (GHR) (A and B), cholecystokinin (CCK) (D and E), gastrin-releasing peptide (GRP) (G and H) and secretin (SCT) (J and K). Positive cells were counted from intestinal sections of controls (n = 6) and mutants (n = 5). Statistical analysis (C, F, I, L) represents the average number of positive cells per crypt-villus axis in each section of the intestine. Error bars represent SE. Scale bar: 50 μm. D: Duodenum; J: Jejunum; I: Ileum. aP < 0.05, bP < 0.001.
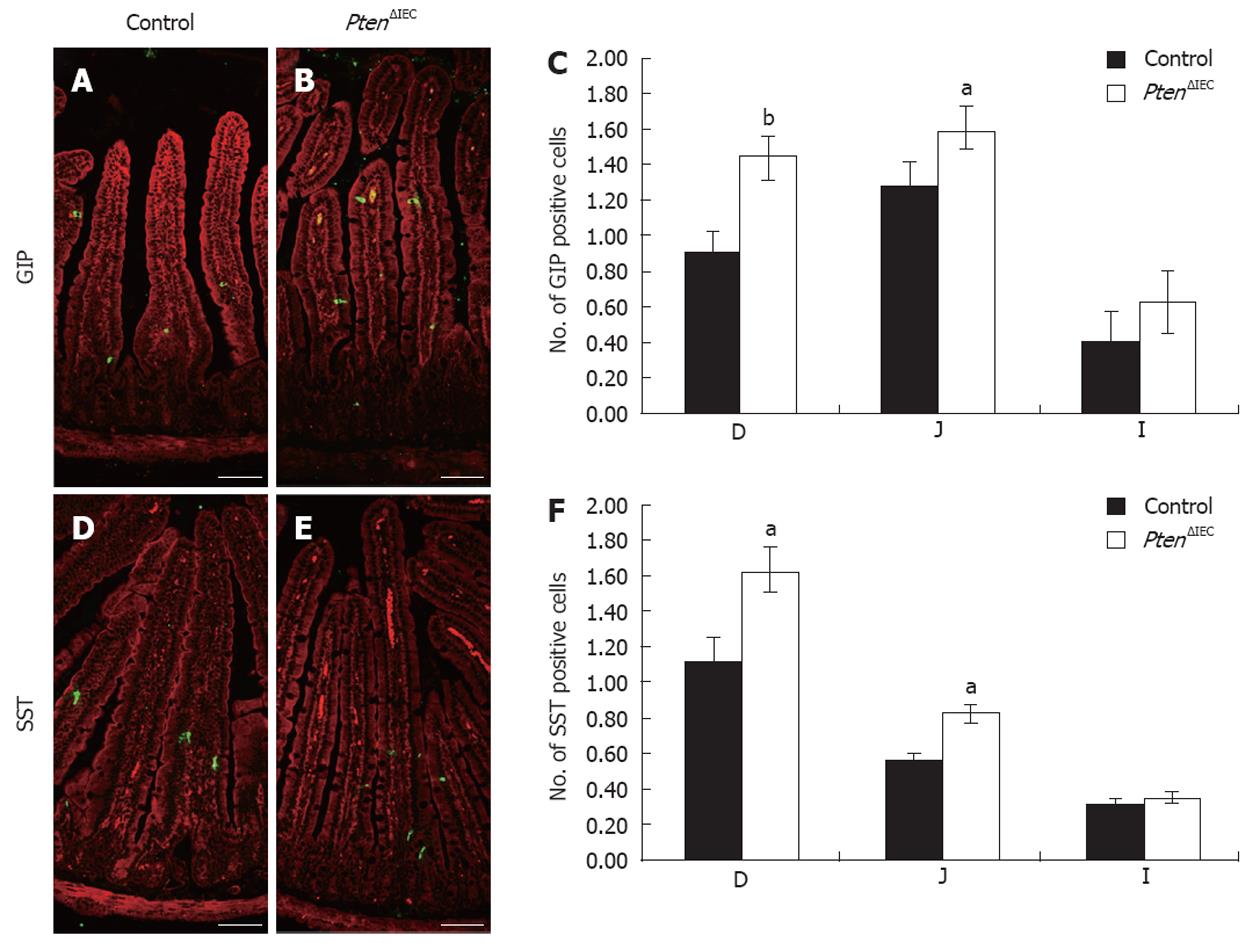
Figure 3 Epithelial Pten negatively regulates commitment of chromogranin A-negative enteroendocrine subpopulations in the small intestine.
Duodenum, jejunum and ileum of adult control and Pten∆IEC mice were immunostained with antibodies against glucose-dependent insulinotropic peptide (GIP) (A and B) and somatostatin (SST) (D and E). Positive cells were counted from intestinal sections of controls (n = 6) and mutants (n = 5). Statistical analysis (C and F) represents the average number of positive cells per crypt-villus axis in each section of the intestine. Error bars represent SE. Scale bar: 50 μm. D: Duodenum; J: Jejunum; I: Ileum. aP < 0.05, bP < 0.01.
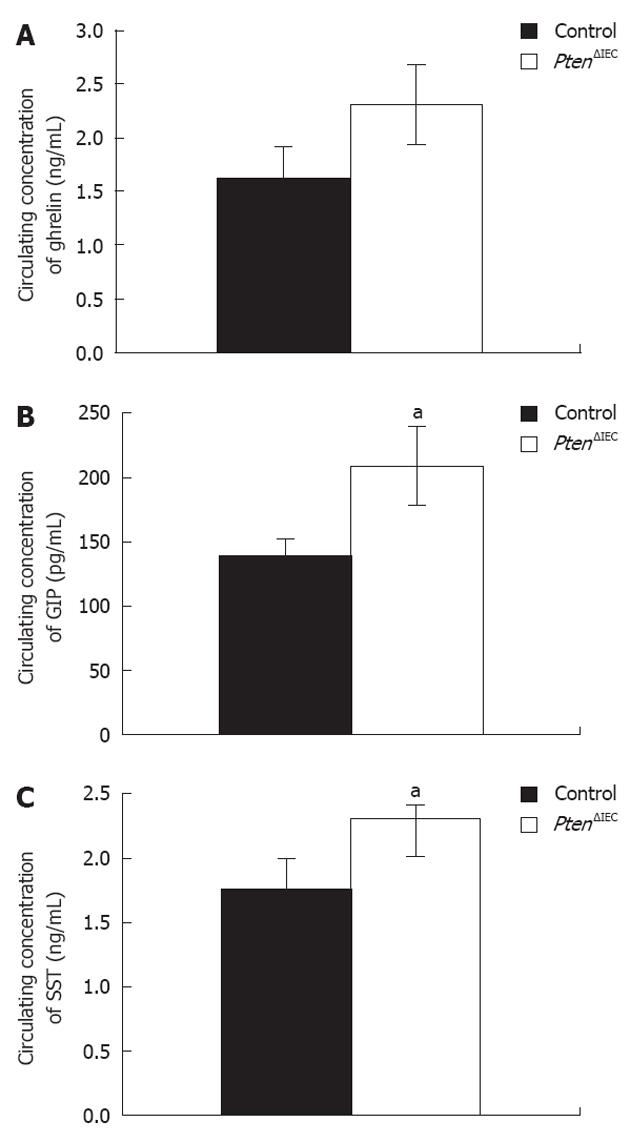
Figure 4 Loss of epithelial Pten signalling modulates circulating levels of glucose-dependent insulinotropic peptide and somatostatin.
A: Analysis of circulating ghrelin level revealed no significant modulation between adult Pten∆IEC mice (n = 10) and control littermates (n = 10); B: Analysis of circulating glucose-dependent insulinotropic peptide (GIP) level revealed a 1.5-fold increase in adult Pten∆IEC mice (n = 10) when compared to control littermates (n = 10); C: Analysis of circulating somatostatin (SST) level revealed a 1.3-fold increase in adult Pten∆IEC mice (n = 10) when compared to control littermates (n = 10). Error bars represent SE. aP < 0.05.
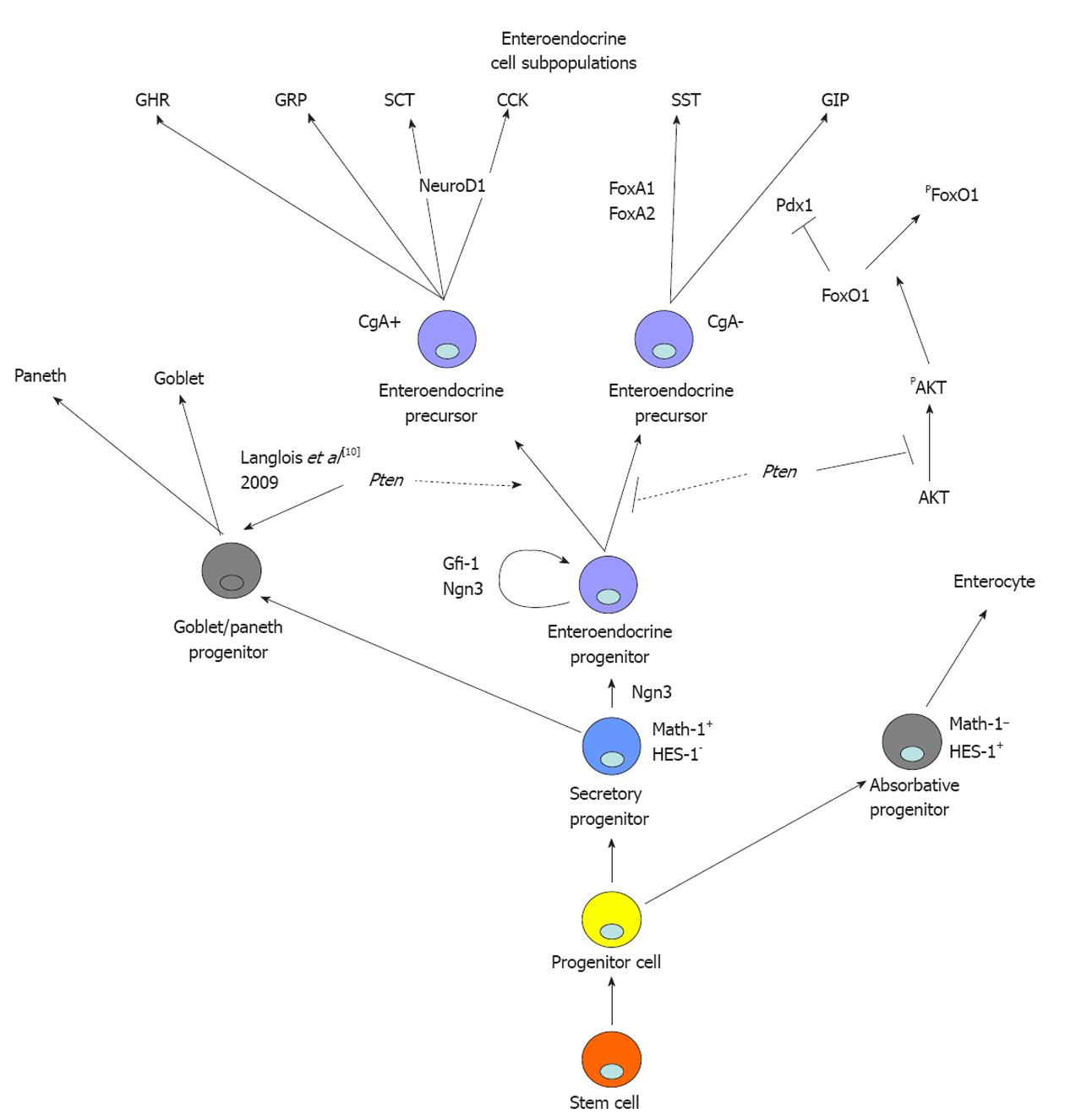
Figure 5 Proposed model for mode of action of epithelial Pten signalling in intestinal epithelial determination and specification of enteroendocrine progenitor cell fate.
Epithelial Pten signalling is not essential for maintenance or determination of the secretory precursor. Pten represses specification of glucose-dependent insulinotropic peptide (GIP)-expressing cells by maintaining FoxO1 in the nucleus. GHR: Ghrelin; GRP: Gastrin-releasing peptide; SCT: Secretin; CCK: Cholecystokinin; SST: Somatostatin; GIP: Glucose-dependent insulinotropic peptide; CgA: Chromogranin A.
- Citation: Roy SA, Langlois MJ, Carrier JC, Boudreau F, Rivard N, Perreault N. Dual regulatory role for phosphatase and tensin homolog in specification of intestinal endocrine cell subtypes. World J Gastroenterol 2012; 18(14): 1579-1589
- URL: https://www.wjgnet.com/1007-9327/full/v18/i14/1579.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i14.1579