Copyright
©2007 Baishideng Publishing Group Inc.
World J Gastroenterol. Oct 21, 2007; 13(39): 5217-5225
Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5217
Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5217
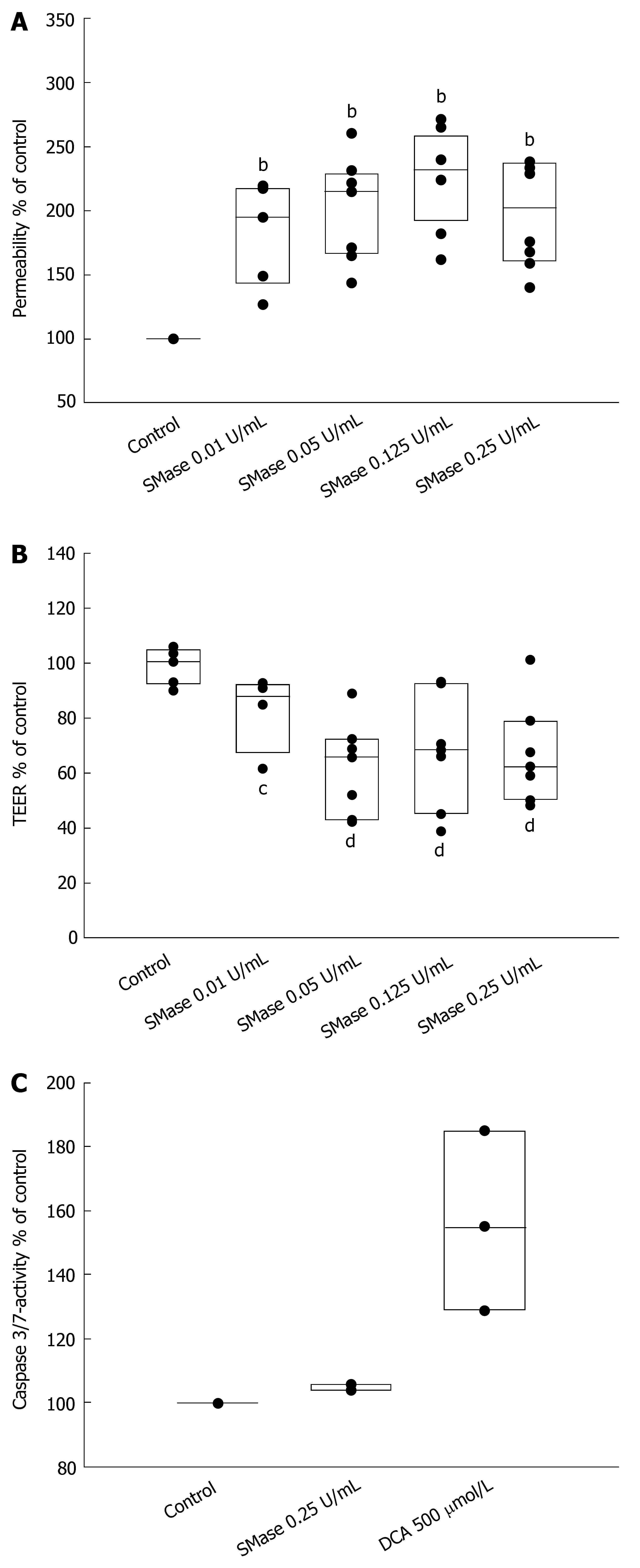
Figure 1 Exogenous sphingomyelinase increases permeability of Caco-2 epithelial monolayers.
A: Caco-2 monolayers were incubated with different concentrations of exogenous SMase. bP < 0.01 between control and treated samples; B: Transepithelial electrical resistance (TEER) was measured across the cell monolayers 4 h after treatment of the monolayers with exogenous SMase. The data were compared with TEER before treatment and are expressed as % of untreated, cP < 0.05, dP < 0.01; C: Epithelial apoptosis was monitored 6 h after incubation with 0.25 U/mL SMase by measurement of caspase-3/7-activity with colorimetric assays. As a positive control, deoxycholic acid (DCA) was used for 1 h.
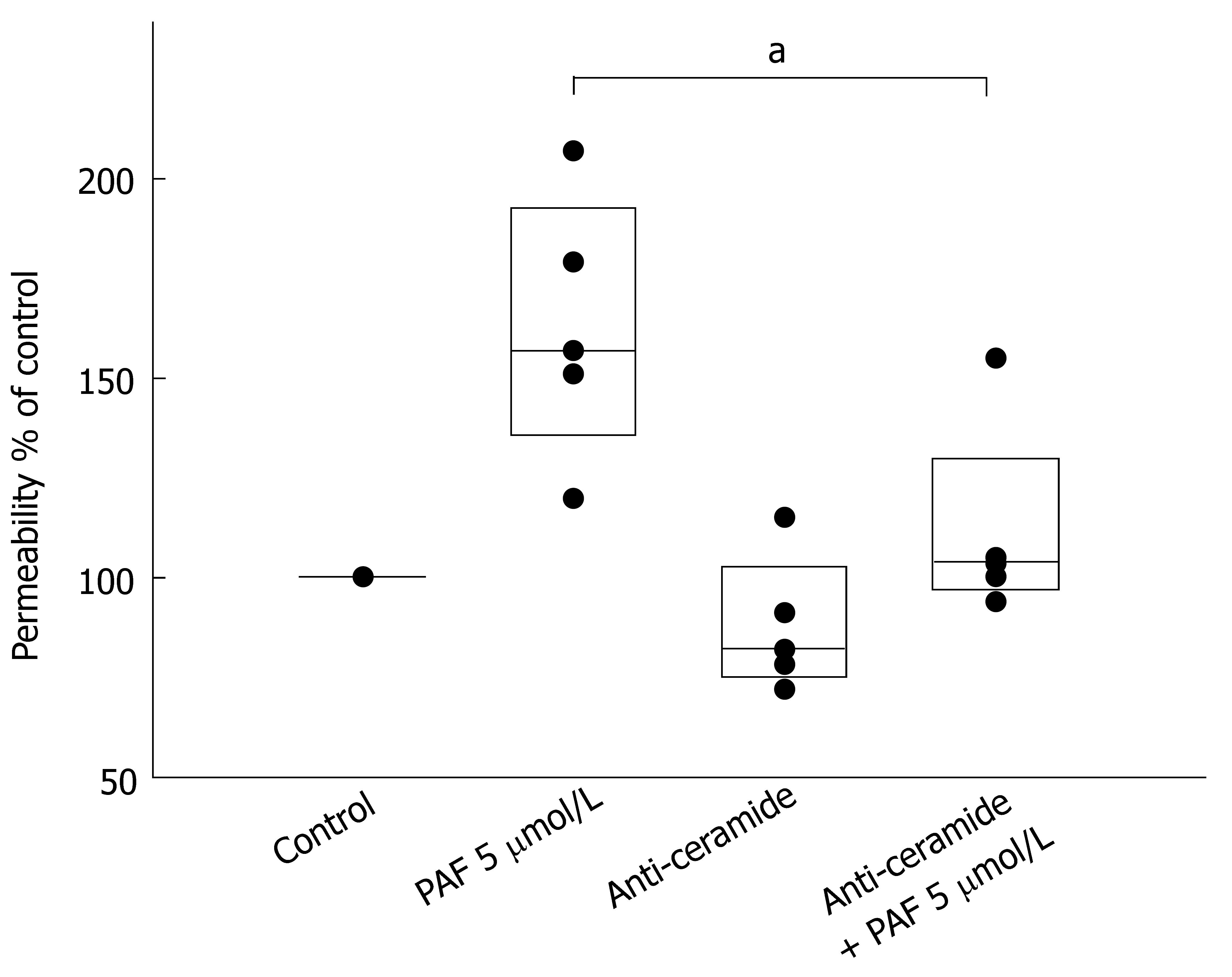
Figure 2 Neutralization of surface-ceramide prevents PAF-mediated increase of permeability.
Caco-2 cell layers were incubated with the IgM ceramide-antiserum (15B4) 30 min prior to stimulation with PAF. Permeability was determined by measurement of transepithelial flux of fluorescein sulfonic acid from the upper chamber of the Transwell insert to the lower chamber across the Caco-2-monolayer after 4 h. The data are expressed as % of control. aP < 0.05 between PAF-treated samples.
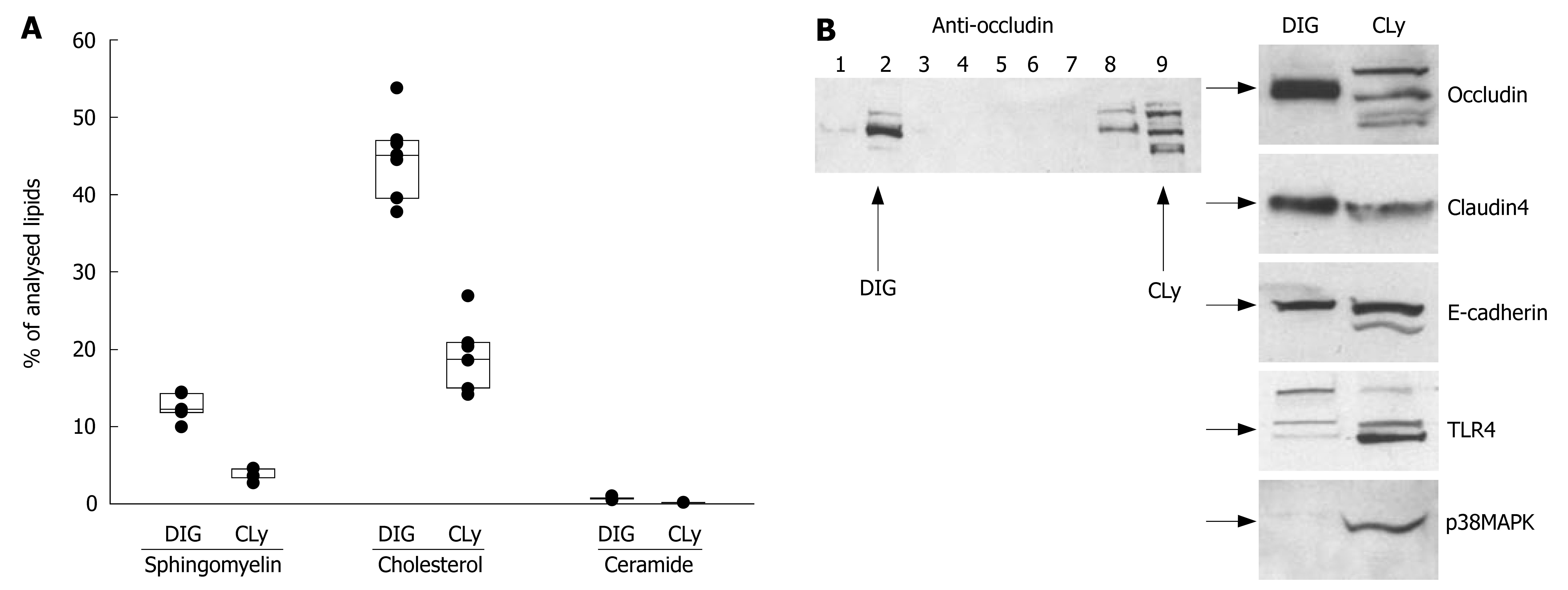
Figure 3 Preparations of detergent insensitive glycosphingolipid-enriched domains (DIGs) contain TJ-proteins.
A: Lipid analysis of DIGs from non-stimulated Caco-2 cells by tandem mass spectrometry revealed the presence of high amounts of sphingomyelin, cholesterol and ceramide when compared to the total cell lysate; B: Gradient fractions were separated by 10% SDS-PAGE and analyzed by Western blot. Fraction No. 2 represents the raft-containing fraction at the 35%/5% interface (DIG); No. 9 represents the soluble fraction (cell lysate = CLy). The presence of high amounts of TJ-proteins occludin and claudin-4 and, to a lesser extent, E-cadherin show a clear association of TJ-proteins with DIGs. The simultaneous absence of other proteins like TLR4 and p38MAPK excludes significant contamination with non-raft fractions.
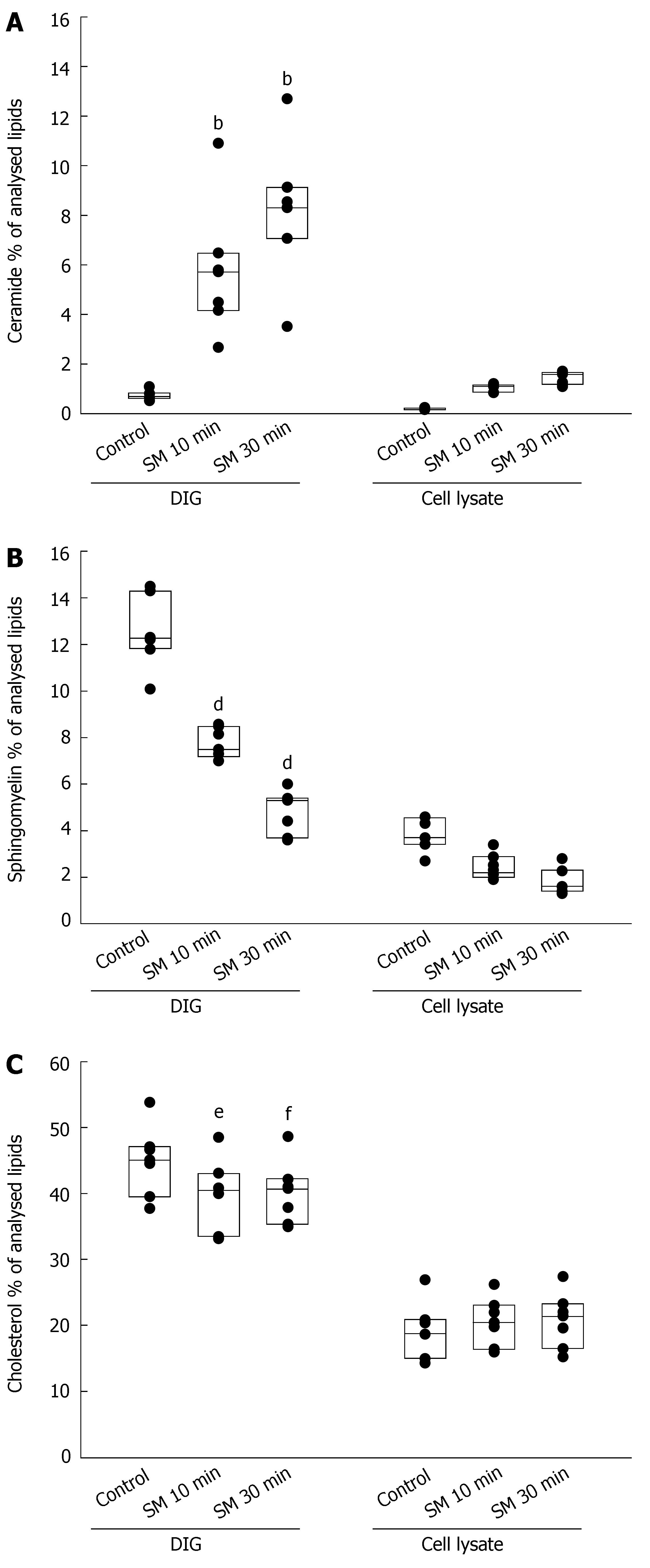
Figure 4 A: Lipid analysis by tandem mass spectrometry revealed a rapid accumulation of ceramide in preparations of DIGs after incubation with 0.
25 U/mL SMase for 10 or 30 min whereas the increase of ceramide in the cell lysate was only small in comparison (bP < 0.01); B: The content of sphingomyelin in DIGs decreased at the same time with a relative reduction comparable to the generation of ceramide (dP < 0.01); C: Cholesterol also decreased in DIGs whereas the concentration in the cell lysate remained constant (eP < 0.05 and fP < 0.01).
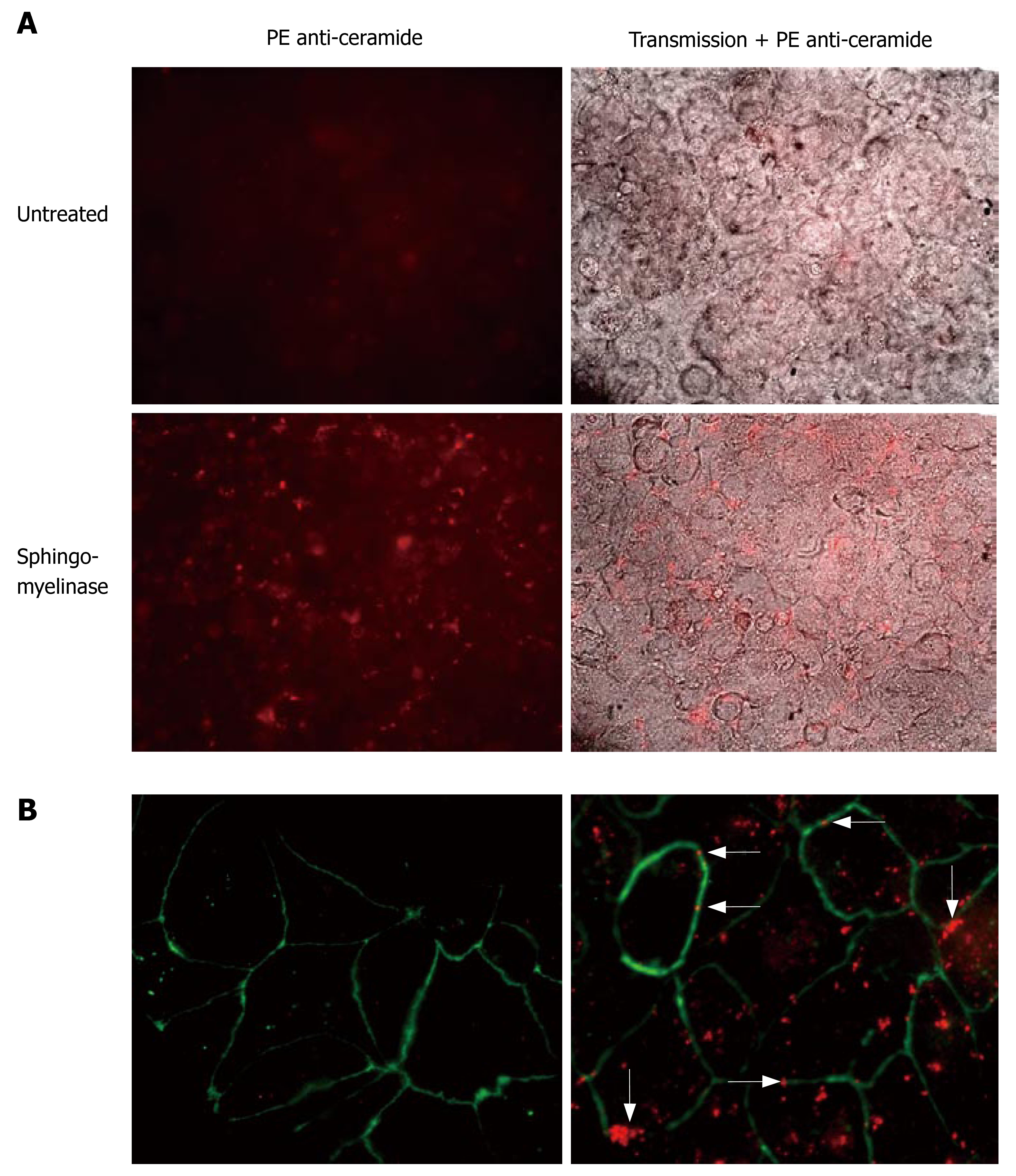
Figure 5 Ceramide clusters at the sites of cell-cell contacts.
Caco-2 cell layers were incubated with 0.25 U/mL SMase for 10 min or left untreated. After fixation of the cells with paraformaldehyde, ceramide was visualized by staining of the cells with the monoclonal anti-ceramide 15B4 antibody and PE-coupled anti-mouse antibodies. A: Top, left: Non-stimulated cells displayed a certain level of background staining that was used to determine the time of exposure. Top, right: Overlay of fluorescence and transmission. Bottom, left: Incubation with SMase resulted in formation of multiple ceramide-clusters. Bottom, right: Overlay of fluorescence and transmission. Close inspection of the clusters revealed a frequent localization of the clusters at the sites of cell/cell-contact; B: Costaining of tight junctions with FITC-labeled anti ZO-1 Abs. Left: non-stimulated. Right: Predominant colocalization of ceramide-clusters with tight junctions upon stimulation with SMase (arrows indicate ceramide-clusters at the sites of cell-cell contact).
- Citation: Bock J, Liebisch G, Schweimer J, Schmitz G, Rogler G. Exogenous sphingomyelinase causes impaired intestinal epithelial barrier function. World J Gastroenterol 2007; 13(39): 5217-5225
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5217