Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 28, 2006; 12(48): 7737-7743
Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7737
Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7737
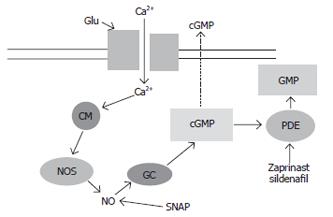
Figure 1 Glutamate-nitric oxide-cyclic GMP pathway.
Activation of ionotropic (mainly NMDA) glutamate receptors leads to increased intracellular calcium (Ca2+) which after binding to calmodulin (CM), activates nitric oxide synthase (NOS), leading to increased production of nitric oxide (NO), which in turn activates soluble guanylate cyclase (sGC), resulting in increased formation of cGMP. Part of the cGMP formed is released to the extracellular space. Soluble guanylate cyclase may be also activated by agents that generate NO such as SNAP. cGMP is degraded by phosphodiesterase (PDE) that may be inhibited by zaprinast or sildenafil.
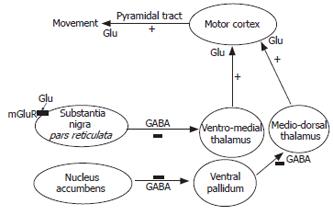
Figure 2 Hypolocomotion in animal models of HE is due to increased extracellular glutamate and activation of metabotropic glutamate receptors in substantia nigra pars reticulata.
Activation of metabotropic glutamate receptors (mGluRs) in nucleus accumbens induces motor activity by activating a neuronal circuit involving ventral pallidum, medio-dorsal thalamus and motor cortex. Activation of mGluRs in substantia nigra pars reticulata (SNr) induces hypolocomotion by activating a neuronal circuit involving ventro-medial thalamus and motor cortex. In rats with HE due to chronic liver failure (portacaval anastomosis), the extracellular concentration of glutamate is significantly increased (15-fold) in SNr, resulting in increased activation of mGluR1 which is responsible for hypolocomotion in these rats. Blocking mGluR1 in SNr with specific antagonists increases motor activity in rats with chronic liver failure up to levels similar to those in normal rats.
- Citation: Felipo V. Contribution of altered signal transduction associated to glutamate receptors in brain to the neurological alterations of hepatic encephalopathy. World J Gastroenterol 2006; 12(48): 7737-7743
- URL: https://www.wjgnet.com/1007-9327/full/v12/i48/7737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i48.7737